
Home / Nuclear Basics / What is radiation?
What is radiation?Radiation is energy travelling through space.Sunshine is one of the most familiar forms of radiation. It delivers light, heat and suntans. While enjoying and depending on it, we control our exposure to it.Beyond ultraviolet radiation from the sun are higher-energy kinds of radiation which are used in medicine and which we all get in low doses from space, from the air, and from the earth and rocks.Collectively we can refer to these kinds of radiation as ionising radiation. It can cause damage to matter, particularly living tissue. At high levels it is therefore dangerous, so it is necessary to control our exposure.While we cannot feel this radiation, it is readily detected and measured, and exposure can easily be monitored.Living things have evolved in an environment which has significant levels of ionising radiation.Furthermore, many people owe their lives and health to such radiation produced artificially. Medical and dental X-rays discern hidden problems. Other kinds of ionising radiation are used to diagnose ailments, and some people are treated with radiation to cure disease.Ionising radiation, such as occurs from uranium ores and nuclear wastes, is part of our human environment, and always has been so. At high levels it is hazardous, but at low levels such as we all experience naturally, it is harmless. Considerable effort is devoted to ensuring that those working with nuclear power are not exposed to harmful levels of radiation from it. Standards for the general public are set about 20 times lower still, well below the levels normally experienced by any of us from natural sources.
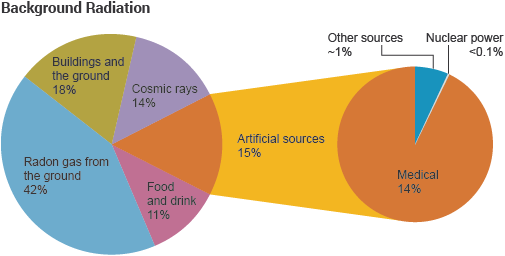
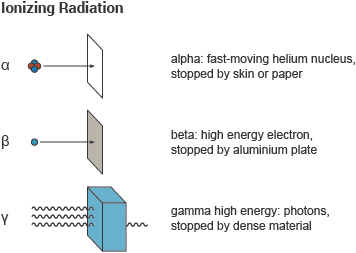
Background radiation is that ionizing radiation which is naturally and inevitably present in our environment. Levels of this can vary greatly. People living in granite areas or on mineralised sands receive more terrestrial radiation than others, while people living or working at high altitudes receive more cosmic radiation. A lot of our natural exposure is due to radon, a gas which seeps from the Earth's crust and is present in the air we breathe.Radioactivity in materialApart from the normal measures of mass and volume, the amount of radioactive material is measured in Becquerel (Bq), which enables us to compare the typical radioactivity of some natural and other materials. A Becquerel is one atomic decay per second, so a household smoke detector with 30,000 Bq contains enough americium to produce that many disintegrations per second. A kilogram of coffee or granite might have 1000 Bq of activity, and an adult human 7000 Bq. Each atomic disintegration produces some ionising radiation.Ionising radiation – alpha, beta and gammaIonising radiation comes from the nuclei of atoms, the basic building blocks of matter. Most atoms are stable, but certain atoms change or disintegrate into totally new atoms. These kinds of atoms are said to be 'unstable' or 'radioactive'. An unstable atom has excess internal energy, with the result that the nucleus can undergo a spontaneous change. This is called 'radioactive decay'.We all experience radiation from natural sources every dayAn unstable nucleus emits excess energy as radiation in the form of gamma rays or fast-moving sub-atomic particles. If it decays with emission of an alpha or beta particle, it becomes a new element and may emit gamma rays at the same time. One can describe the emissions as gamma, beta and alpha radiation. All the time, the atom is progressing in one or more steps towards a stable state where it is no longer radioactive.Alpha particles consist of two protons and two neutrons, in the form of atomic nuclei. Alpha particles are doubly charged (arising from the charge of the two protons). This charge and the relatively slow speed and high mass of alpha particles means that they interact more readily with matter than beta particles or gamma rays and lose their energy quickly. They therefore have little penetrating power and can be stopped by the first layer of skin or a sheet of paper. But inside the body they can inflict more severe biological damage than other types of radiation.Beta particles are fast-moving electrons ejected from the nuclei of many kinds of radioactive atoms. These particles are singly charged (the charge of an electron), are lighter and ejected at a much higher speed than alpha particles. They can penetrate up to 1 to 2 centimetres of water or human flesh. They can be stopped by a sheet of aluminium a few millimetres thick.Gamma rays, like light, represent energy transmitted in a wave without the movement of material, just like heat and light. Gamma rays and X-rays are virtually identical except that X-rays are produced artificially rather than coming from the atomic nucleus. But unlike light, these rays have great penetrating power and can pass through the human body. Mass in the form of concrete, lead or water is used to shield us from them.
The effective dose of all these kinds of radiation is measured in a unit called the Sievert, although most doses are in millisieverts (mSv) – one-thousandth of a Sievert. We each receive about 2 mSv per year from natural background, and maybe more from medical procedures. Anything less than about 100 mSv is harmless.
Related informationNaturally-Occurring Radioactive Materials NORMNuclear Radiation and Health Effects
http://www.world-nuclear.org/information-library/country-profiles/countries-a-f/denmark.aspx
http://www.world-nuclear.org/information-library/country-profiles/countries-a-f/china-nuclear-fuel-cycle.aspx
http://www.world-nuclear.org/information-library/current-and-future-generation/cooling-power-plants.aspx
