Hello everybody! Today, I would like to present to you one of the most important experiments in the history of mankind, performed by possibly the best experimentalist ever lived: Rutherford Scattering. The experiment got its name because it was performed by Ernest Rutherford (and his students) in 1911. Ernest had already been awarded a Nobel Prize in Chemistry in 1908 when he performed his famous scattering experiment. Really, I feel he should have been awarded another one in Physics! Okay, so what was the experiment?
大家好!今天我想跟大家講的是人類歷史上其中一個最重要的科學實驗:拉塞福散射(1911年)。其實在做這個實驗的之前,歐尼斯特.拉塞福已經拿了一個諾貝爾化學獎(1908年)!我個人覺得,在做了拉塞福散射之後,拉塞福是多值一個諾貝爾物理獎的,因為這個實驗的結果實在非常的重要。該入正題了。到底拉塞福散射是什麼東西?

(Rutherford was Director of the Cavendish Laboratory at the University of Cambridge, succeeding J.J. Thomson who discovered the electron in 1897.)
(拉塞福在劍橋大學的物理實驗室當了幾年的老大,在他之前的老大叫J.J.湯姆森。J.J.湯姆森於1897年發現了電子。
The idea of the experiment 實驗的概念
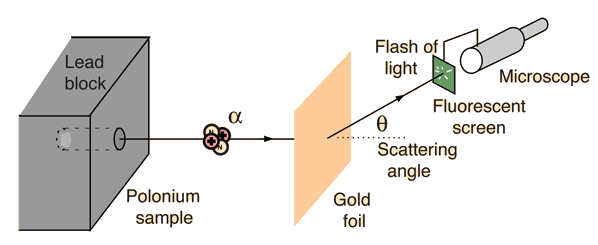
(from hyperphysics)
The set-up of the experiment is illustrated in the figure above. Essentially, we have a source of alpha particles (It isn't important to know what alpha particles are exactly, but to know that they are small and are positively charged. For those who are interested, an alpha particle is a Helium nucleus), which shoots alpha particles at a gold foil. Behind the gold foil is a small fluorescent screen that flashes when it is hit by an alpha particle. The small fluorescent screen can be moved around, so that we know where the alpha particles have gone after hitting the gold foil. The scattering angle that we can detect ranges from 0 to 180.
上圖表達了拉塞福散射的配置:概括而言,我們有一個釋出α粒子的源,把α粒子向一塊純金片射去。在純金片的後面,我們利用一個可移動的小屏幕去探測α粒子擊中純金片後往哪兒跑了。探測的角度(scattering angle)由零到一百八十都可以。(我們不用知道什麼是α粒子:我們只要知道它很小,還有是正電極的就可以了。)
The result of the experiment 實驗的結果
One might think: surely if alpha particles will get stopped by the gold foil just like how if we can't run through a wall? This might be what Rutherford was thinking as well, but Nature likes to surprise us: Most of the alpha particles went straight through. You might think: Wow, what's going on there? The interesting is yet to come: Some of the alpha particles (1 in 8000) were backscattered, meaning that they basically bounced back to where they came from.
我們可能會覺得:α粒子會被金片停來下吧,畢竟如果我們往着牆跑會撞的很慘。可能拉塞福自己也是這麼想的, 可是大自然總喜歡告訴我們:你們人類其實什麼都不懂。結果出來:絕大部分的α粒子完全無視了金片的存在。這還不算最有趣的:很少部分的(大概每八千次有一次)α粒子基本反彈回去了。

Rutherford was so shocked by the results that he reacted with what is now a famous quote: "It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you!"
If you don't know what a 15-inch shell look like, here it is:
拉塞福完全被這個結果嚇倒了。他的反應是:這太神奇了,就好像你把一顆十五吋的炮彈射向一張紙,然後那炮彈反彈回來打中你了!
要是你不知道一顆十五吋的炮彈長什麼樣,這就是了:

Rutherford's interpretation 拉塞福的結論
So how did Rutherford make sense of his bizarre results? His resolution was: (People were aware that things were made up of atoms in those days, so a gold foil would just be a sheet of gold atoms. What they didn't know was what the atom looked like)
- Most of an atom is empty space, hence most of the alpha particles could go straight through the gold foil.
- The atom has a very dense and positively charged region that is responsible for the backscattering: what we now know as the nucleus of an atom.
Rutherford's findings directly contributed to our understanding of atomic structure today. Today, we know that the nucleus of an atom is made of protons and neutrons. Protons are positive while neutrons are chargeless, so the nucleus overall is positively charged.
聰明的拉塞福如何去理解實驗的結果呢?他作出了以下的結論(當時的科學家們已經知道原子是萬物構造成分的基本,但是他們不怎麼知道原子長什麼樣的。所以他們是知道一塊純金片基本上就是一大片金的原子。)
- 原子的大部分都是空的,所以大部分的α粒子基本沒有發現金原子的存在。
- 原子裏有一個特別小,密度非常高的,正電極的地方。就是這個地方把那些極少數的α粒子反彈回來的。今天我們知道這地方就是原子中的原子核。原子核由中子及質子組成。質子是正電極的,中子是沒電極的,所以原子核就是正電極的。
拉塞福的發現為人類今天對原子結構的認知有着非常重大的貢獻。

In the picture above, the red balls are protons, while the blue balls are neutrons. The grey balls are electrons (discovered by J.J. Thomson); they are negatively charged. In an atom, the number of electrons and protons are always the same so that overall the atom is electrically neutral.
在上圖,紅色的是質子,藍色的是中子,灰色的是電子 (沒錯,就是J.J.湯姆森發現的電子)。電子是負電極的。在原子裏,質子跟電子的數量是一樣的,所以原子是沒電極的。
If you have patiently read all of the above, congratulations! You now know how us humans know what the atom looks like! And of course, thank you very much for reading and I hope you have enjoyed this interesting (true) story. I am hoping this will develop into a series (Hence this is called Great Experiments (1)). I am also thinking of starting other series, perhaps some Quantum Mechanics or Relativity? Or Astronomy? Any suggestions are welcome!
如果你有耐心讀到這裏,太好了!你現在知道我們人類是怎樣學到一個原子是長這個樣的。當然,非常感謝你你把我的文章讀完:)希望你喜歡這個有趣的真人真事。這篇文章的名字是「偉大的實驗(一)」。我希望能慢慢的寫出一個系列來,也正在構思其他的系列,比如量子力學,相對論,天文學等等。歡迎給點意見!
