Click the small images for fun
"Dark chocolate is essentially a Warm fluid mass of cocoa butter that contains suspended particles of cacao beans and of sugar. The last step in manufacturing chocolate is too cool the fluid chocolate to room temperature and form the familiar solid bars" Harold McGee, Food and Cooking.
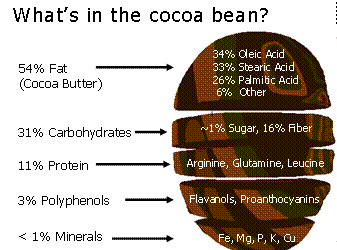
The different types of chocolate come all from different proportions and mixtures of this ingredients. For instance dark chocolate is 60% cocoa paste, 30% sugar and 10% cocoa butter. Milk chocolate is 12% cocoa paste, 28% cocoa butter, 40% sugar and 20% powdered milk. White chocolate 30% cocoa butter, 45% sugar and 25% powdered milk.

Some of the main chemical compounds that give chocolate their flavor and effects are Phenethylamine, Theophylline, Catechins, Caffeine, theobromine. The flavor is also influenced by other power sources, the texture which adds more than just haptic complexity to the taste. This texture comes from subtleties in the phase transition of the structure in chocolate.
The correlation of textures goes from chocolate mousse, that feels soft like a foam, to soft chocolate candy bars to chocolate chunks hard as rocks.
What determines the mouthfeel is the elasticity. Think of a spring. If you put a weight on top, it will shorten in length. If you put a weight at the bottom of one extreme with resistance at the other it gets longer by a certain length. The degree at which this delta occurs is given by the Hooke's law.  , where F is the force in Newtons, k is the spring constant given in Newtons per meter and the Delta of L is the displacement in meters.
, where F is the force in Newtons, k is the spring constant given in Newtons per meter and the Delta of L is the displacement in meters.
If you want to measure the elasticity of material you measure force and displacement, but since is a block of chocolate you don't want to worry about uniformity, so you normalize it, this is dividing the force by the area. Which gives you the stress.
Simmilarly, when you displace something you want to treat it uniformly regardless of size, so you calculate the relative displacement by dividing the Delta of L by the initial length, called the strain. 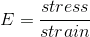
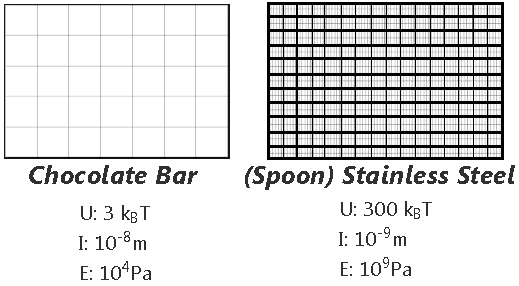
The molecular structure can be thought of as being connected by springs. The overall elasticity of the crystals depends on the bond strength or energy density (U) and the tridimensional bond spacing between molecules (I). 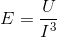
This is the structure of a chocolate bar compared to steel.
The process that allows the selection and change in the structure of chocolate to acquire complex textures is called tempering, that is a form of phase transition control.
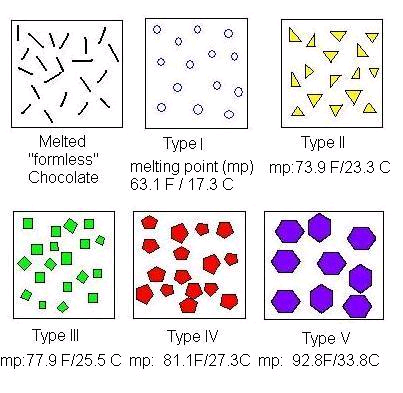
A critical point in phase transition is a well-defined temperature at which it changes phase. This is very important in chocolate. Most materials have several phases. The best example is water. Acording to the kinetics of phase transition. As you cool something down, the molecules go from a very disorderly, highly movable state to a locked position where they form a crystal-like structure. This solidification can take quite a long time. In case you don't know, most likely you do. Water can go below the freezing point (0 °C/32 °F) without turning into a solid, this is done by stopping the apparition of nucleation centers. Nucleation is the first step in the formation of a new thermodynamic phase that determines how long an observer needs to wait before a self-organized structure appears. First order transition. It can also appear in other interfaces like when an object falls into soda and bubbles form around. Controling Nucleation is something that must be done precisely with chocolate. Normally, nucleation is homogeneous as it occurs randomly across space. In the case of supercooled water, we see heterogeneous nucleation as the symmetry breaks at that point The melting behavior depends on the composition. The main component in chocolate is the cocoa butter, like all of the fats it has a variety of different temperatures. The reason for that is that there are different molecules with different orientations that can arrange themselves in different crystal structures. So during a process called tempering, what chocolatiers do is control the rate of temperature lowering so that a lot of different crystal structures are formed. Not all of them is desirable, so you raise the temperature to get rid of the ones you don't want, and the ones that still remain, those work as heterogenous centers of nucleation to force that specific crystal structure in chocolate to grow. If tempered, cocoa butter solidifies relatively quickly ( If you have ever experienced have a chocolate bar melt during a hot day, later to have it solidify again by putting it in the freezer. What you get is all the chocolate crystal forms and the taste and feeling are affected as in structural composition are like tasting dirt. That's what happens when the changes are too abrupt and what tempering avoids Normally Chocolatiers melt the chocolate at around 45 °C to make sure all the crystals forms are gone. They work mainly with forms V and VI as they are the most stable. What they do is lower the temperature abruptly to 28° C, below the form V and slightly above the form IV. Despite that, the relative change in temperature creates other crystal forms, so to destroy them chocolatiers augment the temperature to 31°C. In this form only the crystals of the form V remain and will become the crystallization nuclei.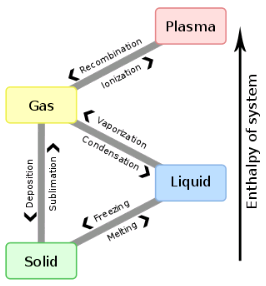

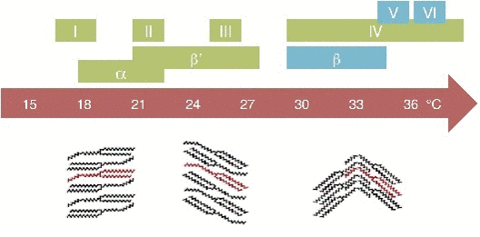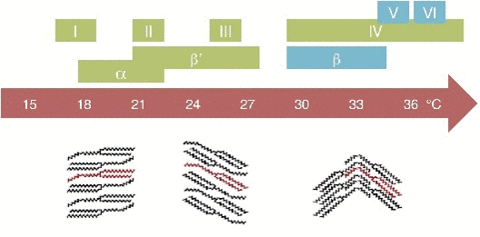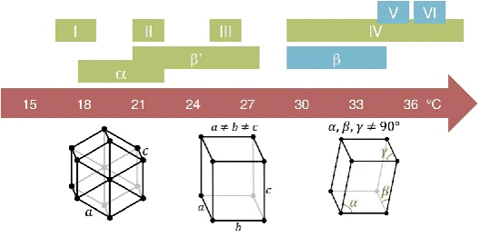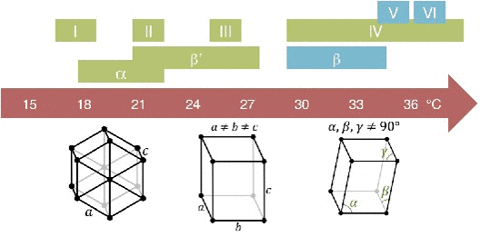
 20 minutes), if untempered it takes around 3 days to solidify. The textures are different. If tempered the structures is regular and if untempered is irregular.The cocoa butter is formed by tryglicerides (glycerol+fatty acids S,O,L)
20 minutes), if untempered it takes around 3 days to solidify. The textures are different. If tempered the structures is regular and if untempered is irregular.The cocoa butter is formed by tryglicerides (glycerol+fatty acids S,O,L)
Triglycerides
Melting Point
Stability
I (gamma)
16-18°C
Very unstable
II (alfa)
22-24°C
Unstable
III (beta 2')
24-26 °C
unstable
IV (beta 1')
26-28 °C
unstable
V (beta 2)
32-34°C
Stable
V (beta 1)
34-36 °C
Stable
As you might remember, heterogeneous crystallization is far quicker than homogeneous crystallization. So when the temperature goes quickly <20 degrees all chocolate solidifies in the form of the V and VI. Total stabilization and takes weeks. The bigger crystals diffuse light and give it a gey appearance and lack of gloss. This is undesirable. the form VI causes contraction of the chocolate and the fat to migrate closer to the VI nuclei that form in the surface. Due to their big size, they take longer to appear and only form in relatively sparse quantities in the beginning, despite their melting point.
The size of the crystals also has a result in the flavor. Smaller crystals will take a shorter time to break and liberate the flavor. What happens with chocolate as a beverage. Some emulsions are used to preserve small sized crystals like in mousse by obtaining the elasticity from other ingredients
References
Images: Sources included, otherwise linked to original source or modified from google images labeled for reuse



