TL;DR It seems there are genetic regulatory pathways for aging. Some individuals have expressed mutations that favor maintenance and longevity. In normal individuals environmental stress particularly caloric restriction seems to inhibit those pathways leading to more longevity.
One of the great notable differences about animals of the same size is their lifespan. A mouse is similar in size and shape to a bat but the bat's lifespan is 25x that of the mouse. On the other hand, how does an animal as big as a whale reach 200 years? The reason for this seems to be that they have different genes.
If you take an animal with a relatively short lifespan whose DNA is well known by researchers then if in the same species there are longevity differences those must have alterations. This seems easier than comparing one animal to another. The logic of this is that if there are points of regulation in genes for aging, then producing an animal that could live longer is possible.
This was tested in a worm called C. elegans. Their lifespan is close to two weeks after hatching. It didn't take long before a mutant variation that already lived to 30-50% longer than a normal worm was found.
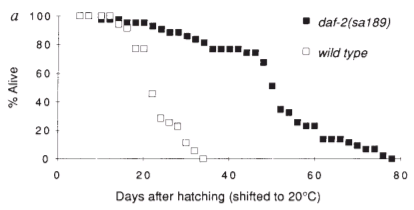
A mutation identified like DAF-2 doubled the lifespan of the worms that had it.1 As shown by the graphic, by day 30 in the wild type are all dead but the in the mutant above 80% are still alive and thriving at the same time.
To put it into perspective. A single mutation made the worm equivalent of a 90-year-old human behave like one in its late 20's.
The DAF-2 gene encodes a hormone receptor, is a membrane receptor and its stimulation speeds up aging. This receptor is similar to two hormone receptors in humans: The receptors for insulin and IGF-1 2 these receptors signal the uptake of energy and growth.
This actually correlates well to other animals. In the case of Drosophila melanogaster3 a mutation in the gene that encodes the insulin/IGF-1 receptor results in higher longevity, which can be taken as a hint that the normally functional receptor acts as an inhibitor of longevity.
In the case of mice, the receptors are separate. If a mouse is heterozygous for the mutation of the IGF-1 receptor it still lives 20% longer. If you remove the functionality of the insulin receptor from the fat tissue the mouse lives longer and even with a high-fat diet they don't get fat. 4
This means is a highly likely scenario that they share a common ancestor in which this was the mechanism for controlling aging. This path can be traced further.
In the case of dogs, it has been shown that a big dog like a great Dane can live 5-7 years, while a small dog like a chihuahua can live up to 20 years (a difference of 50 times in mass for the same species). The single determinant factor for size is a single allele difference in IGF-1.5

The effect of the homologous genes to DAF-2 have different periods of activity. In the case of Drosophila and C. elegans, there was no alteration to size to extend lifespan and since growth in most animals is not required to continue beyond a certain period. In the case of C. elegans the turn down of the DAF-2 gene by RNAi at different stages of its life and found that most of the benefit comes from turning the gene down after adulthood.6
Another factor identified was the presence of DAF-16 that acts as a transcription factor. In DAF-2 mutants that also lack DAF-16 the extension of lifespan is no longer functional.7
DAF-16 encodes a transcription factor that is at the end of a kinase cascade, and when phosphorylated, DAF-16 accumulates in the nucleus and seems to be the center of a cumulative cascade of regulation in other nuclear genes.
In the case of mutants with alterations for the path of IGF-1, like GH mutations, there are no significant benefits to caloric restriction as the route is already down-regulated. In mice without the alteration the caloric restriction makes them behave like the mutant.8
These genes seem to be an evolutionary adaptation to changing environmental conditions and means to protect the population enough to reproduce and to age shortly after resources have normalized and reproduction has been fulfilled.
As a side note: In another post, I examined briefly a type of caloric restriction diet known as intermitent fasting. There I mentioned how caloric restriction diets are the only reliable known way, applied statistically to populations to extend life. In the case of humans the official position is that concensus is that the long term effects are unknown9 , the benefits of this are controversial in their universality in most animals since there exist variation in response for individuals that have a genetic makeup that has alterations in this pathway.
References

Images referenced, sourced or modified from google images, labeled for reuse
My previous post ↶ @ertwro