Purpose
To get information about solubility of salts and determination of dissolution temperature.

Theoretical Knowlodge
When two different pure substances are mixed and a homogenous is obtained, the temperature of the mixture is generally different from the temperature ( at the same time ambient temperature) that before mixing the pure components. Therefore, heat exchange occurs between the mixture and the environment. According to Hess’s Law, amount of heat exchange between system and evironment is,
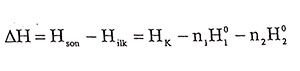 (1.1)
(1.1)given by this equation. HK in the equation is total enthalpy of the mixture, as for that H01 and H02 are the molar enthalpies before the components were mixed. Enthalpy equation for homogenous mixtures of two components is,
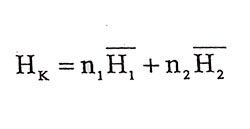 (1.2)
(1.2)given by this form. H1 and H2 are partial molar entalpies of related components in the mixture. If calculate differential while n1 is constant to n2 and while n2 is constant to n1 at constant temperature and pressure, respectively,
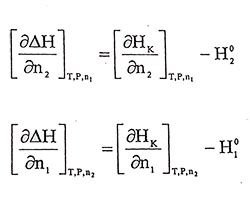 (1.3 - 1.4)
(1.3 - 1.4)Equations are obtained. In equation 1.3.
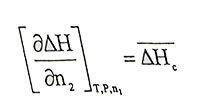 (1.5)
(1.5)is differantial molar dissolution or partial molar dissolution entalphy, it is the enthalpy exchange when one mole of pure soluble is added to solution in such an amount that the concentration of the solution does not change. In equation 1.4,
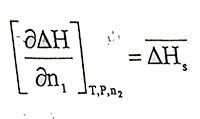 (1.6)
(1.6)is differantial molar dilution or partial molar dilution enthalpy, it is the enthalpy exchange when one mole of pure solvent is added to solution in such an amount that the concentration of the solution does not change.
Taking into account equation 1.2, 1.3 and 1.5,
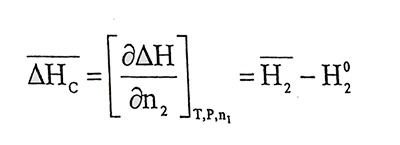 (1.7)
(1.7)And also taking into account equation 1.2, 1.4 and 1.6,
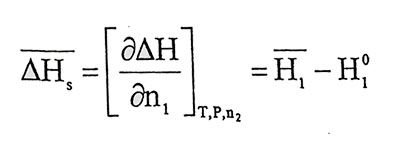 (1.8)
(1.8)Equations can be written. If equation 1.1 and 1.2 combined each other,
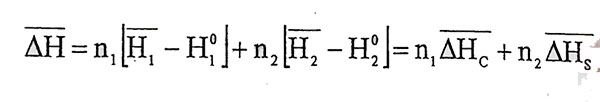 (1.9)
(1.9)Equation is obtained.
Integral dissolution enthalpy is enthalpy change per unit mole of solvent after mixed n1 mole solvent and n2 mole soluble at constant temperature and pressure. Considering this definition, from equation 1.1 and 1.9 for dissolution enthalpy,
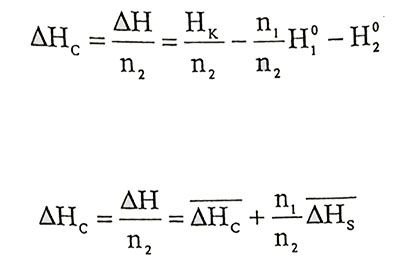 (1.10 - 1.11)
(1.10 - 1.11)Equations can be written. ΔHC that dissolution enthalpy will be changed with temperature because of ΔHC and ΔHS are function of temperature. The effect of temperature can be neglected in the low temperature range.
Temperature Dependence of the Resolution
Solubility is the saturation concentration. As to saturation concentration, in a spesific temperature and pressure, is concentration of extra soluble matter that in equilibrium. When equability established for saturated solution which in aqueous solution of solid salts, equilibrium constant, that K
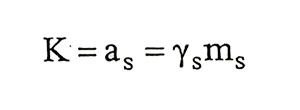 (1.12)
(1.12)given by this equation. In the equation, a is activity, y is activity factor and m is molal concentration. s subscript shows saturity condition. Solubility of solid salts changes due to temperature and Van’t Hoff isochoric that,
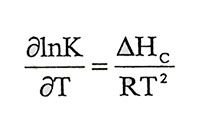 (1.13)
(1.13)Enonciable this equation. Consideringly if ΔHC is constant and y is constant near the saturation concentration, with integral of equation 1.13
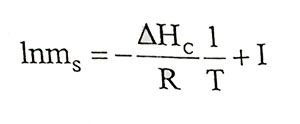 (1.14)
(1.14)Equation can be obtained and can be edited as follows for two temperature range:
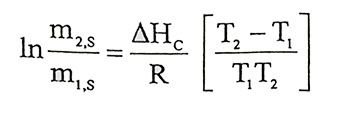 (1.15)
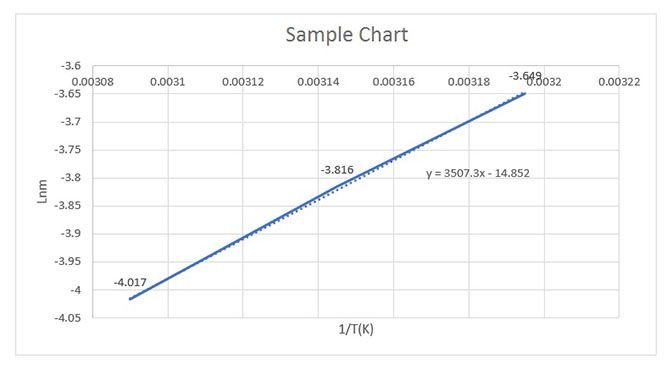
(1.15)Salt resolution is calculated at different temperatures and that calculated values can be used for graph that 1/T to lnms. Heat of solution can be calculated by slope of the line segment that will be obtained from graph.
References:
- Petrucci, Ralph H. ,(2010), General Chemistry 10E : Principles and Modern Applications with MasteringChemistry, Pearson
- https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/
- Eskisehir Osmangazi University, Department of Chemical Engineering, Physical Chemistry Lab. Brochure
