
Several years ago I saw Val H. Smith (no relation) present ideas about the ecology of the human bloodstream. I’m pleased to know that he continued this line of research. For example, this paper, Host nutrition and infectious disease: an ecological view gives a broader perpective than what he presented then.
Ironing out the dynamics of bacterial infections
What I remember most from that presentation several years ago was Val explaining the importance of iron for blood-born bacterial infections. We all need iron as an essential component of the hemoglobin that our red blood cells use to transport oxygen, and for other metabolic functions as well. Iron is also an essential nutrient for many other life forms, including certain bacteria that can cause life-threatening infections.

A diagram showing the structure of hemoglobin.
We need iron, but in the right places
Iron is common in many vitamin pills and dietary supplements, particularly for women. However, taking in too much iron can cause serious health problems, particularly for adult men and women after menopause.
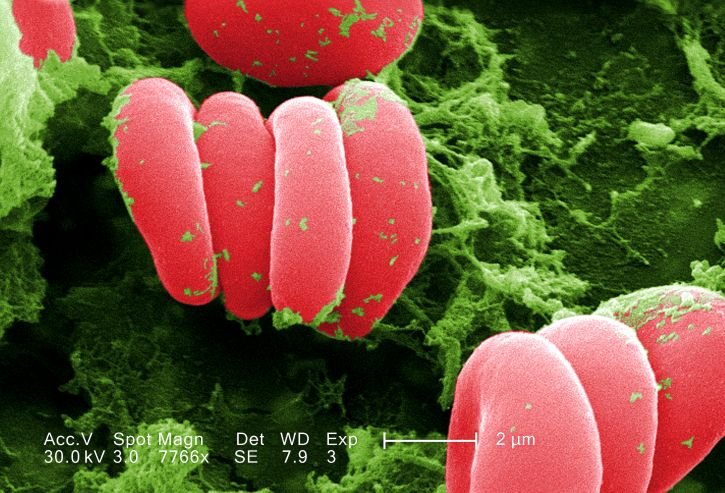
Red blood cells.
Although we need sufficient iron to transport and use oxygen, it’s also extremely important that only very little iron is freely dissolved in our blood plasma (the liquid). In a healthy human, most iron is held inside our cells or bound tightly by special chemicals (called chelating agents). One reason that our bodies keep the free iron concentration in our blood so low is so that invading bacteria will not have ready access to the iron that they need to grow and prosper. Not only humans, but all mammals have elaborate systems for regulating iron concentrations in blood and tissues.
Bacteria and plankton need their iron, too.
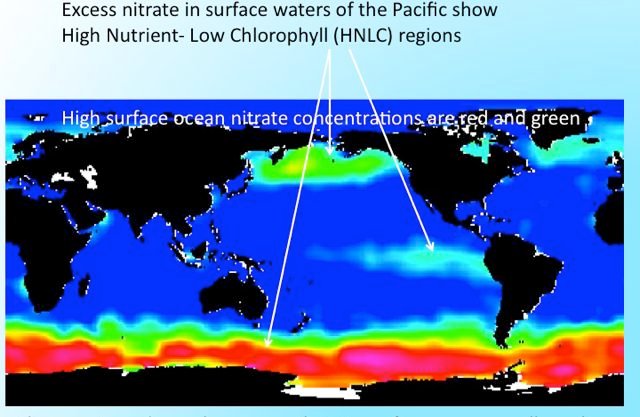
Bacteria, and many algae, need iron for various enzymes, which are essentially catalysts that facilitate (greatly speed up) the many chemical reactions necessary to support life. It has been recognized for at least 20 years now that the productivity of large areas of the ocean is limited by low iron concentrations. Such areas are called High Nutrient Low Chlorophyll, or HNCL regions. Because of insufficient iron, the algae are not able to use very much of the other nutrients like nitrogen, which accumulate in the water. It was a mystery for a while why the algae were not growing, despite plenty of nitrogen and other nutrients. Then it was understood that iron was also a vital nutrient for the algae.
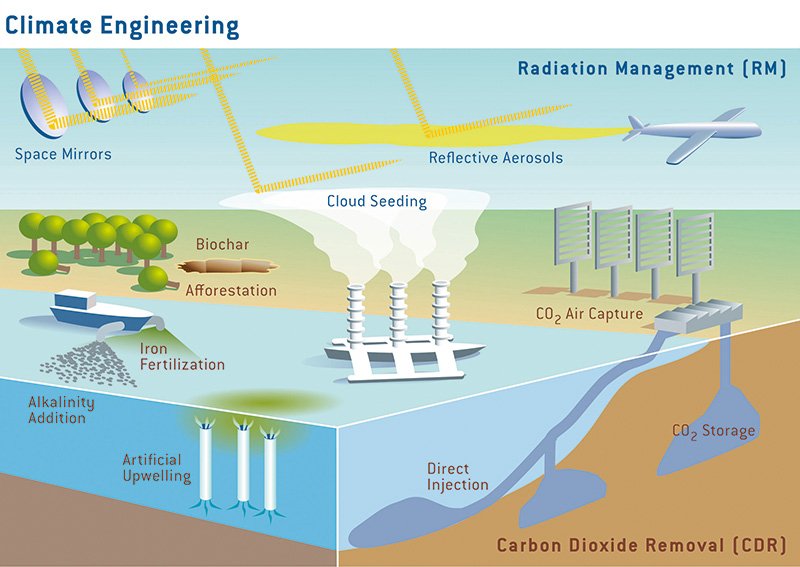
Fertilizing the ocean with iron has even been suggested as a form of geo-engineering in order to increase the productivity of plankton and thereby enhance the removal of carbon dioxide from the atmosphere. (As the algae grow, they convert inorganic carbon, essentially dissolved CO2, into organic carbon.)
Why chicken soup is good food for the sick
Back to humans and our diseases: I learned this from Val Smith’s presentation. Chicken soup provides valuable nutrients, with less iron than red meats such as beef. This helps sustain the body without providing so much iron as to make life easier for invading bacteria, which is important when the body is weakened by a cold or infection.
Tetanus: not an iron-clad case
Tetanus, caused by the bacterium bacterium Clostridium tetani, is probably the best-known example of a dangerous infection that can occur from a cut or scrape on rusty metal. Although it is commonly thought that the iron causes the infection, tetanus is not exclusively caused by contact with rust. We can get tetanus from soil, feces, scratches from animal claws, etc. Any wound that gets infected and provides a place for this anaerobic bacterium to produce its toxins can lead to the disease.
Mutations that confer resistance to malaria while increasing risks of other diseases
The story of Human genetic resistance to malaria is a fascinating lesson in how natural selection weighs the costs versus benefits of different gene variants. The parasites that cause malaria invade and feed on human red blood cells. However, individuals having even very slight mutations (sometimes just one DNA base is different) in the genes coding for hemoglobin and other proteins are much less susceptible to malaria. This is because even slight changes in protein structure (for example, just one in a long chain of amino acids being different) can change the structure and function of the globin component of hemoglobin. This can make it much more difficult for the parasites to feed off of the host cells, or make the infected cells clump together less, or make it easier for white blood cells to detect and neutralize infected red blood cells.

Even genes that cause harmful diseases can be quite advantageous in certain environments
Malaria resistance is the oldest and perhaps best example of this. In areas where malaria has been common for a long time, as much as 40% of the human population has some such mutation that confers resistance. Probably the best known disease resulting from such a mutation is Sickle Cell Anemia, which alters the shape of the red blood cells. The human populations native to drier more temperate climates have virtually no such mutations, because they are of no advantage in the absence of mosquitos and malaria. Clearly there is very strong selection pressure in favor of such disease causing mutations for populations living with malaria.
Rest in Peace, Val Smith
While searching for more information to write this post, I learned that Val Smith passed away a few months ago. Condolences to his family and friends. I enjoyed his presentation and his publications, which we still have very much to our benefit. I dedicate this post to the memory of his scientific achievements.
S. Lan Smith
Kamakura, Japan
September 20, 2016
Thanks to everyone who produced and made freely available the images that I used in this post.
