The Problem
For the last several years, there have been concerns about estrogen in our water. The sources are very diverse, untreated animal waste, soil, with other sources being investigated. According to this article, published in the Journal of the American Chemical Society, the contraceptive we commonly refer to as “the pill” contributes roughly 1%.
https://www.acs.org/content/acs/en/pressroom/presspacs/2011/acs-presspac-february-23-2011/new-report-dont-blame-the-pill-for-estrogen-in-drinking-water.html
While the sources are not yet all identified, it cannot be denied that we can find estrogenic compounds in surface waters, such as lakes and rivers. Today I want to take the chance to introduce my research, which has the goal to develop novel water filters that can filter out estrogenic compounds.
But first, why do we worry about estrogen in our water?
Estrogen is an endocrine hormone, meaning that is it released in one part of the body by a gland, and then transported to another part of the body by our circulatory system. At its destination, estrogen binds to selective receptors, which then cause a complex system of cellular mechanisms to occur. Discussing these would go beyond the scope of this post. Estrogen has many effects and can affect the development of sexual organs, and is a possible cause of cancer (however, this is more a hypothesis and needs more research to confirm or deny this statement). So, while the estrogen release is controlled by a complex system in our bodies, intake of external estrogen, or estrogen-like compounds, can have potentially harmful effects. These negative effects can be seen in nature already, for example fish. In short, estrogen can change genes and mineralized structures in fish.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4145326/
https://www.lunduniversity.lu.se/article/oestrogen-in-birth-control-pills-has-a-negative-impact-on-fish
There is evidence suggesting that the estrogen found in drinking water is not a problem for humans. The estrogen that accumulates in fish might be a bigger problem to humans. There is no imminent danger as of now, however, estrogen in water is a phenomenon to be observed and research to prevent rising levels of estrogenic compounds in our water is important.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2854760/
Fungus to the Rescue

As mentioned earlier, my research focuses on the development of filters that are specific to estrogenic compounds. Nature served as the inspiration. The fungus trametes vesicolor (Tv) which feasts on dead trees, developed an enzyme that helps it to break down lignin, which is the chemical that makes wood very rigid by tightly “gluing” together cells. This enzyme is called Tv laccase (TvL). Where is the link? Lignin has a functional group, called phenol, just as estrogenic compounds.
Laccase is able to oxidize phenolic groups, creating an oxygen radical at the phenol group, which then causes polymerization of the phenolic substances. This leads to polymers which, after reaching a critical length, precipitate out of water.
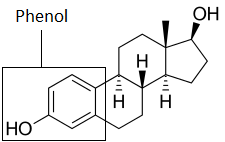
My work focused on the characterization of phenolic substrates for TvL. I found substrates by exposing potential substrates to a solution that contained a pre-determined concentration of laccase (1.5 micro molar). After a day, I spun the solutions down in a centrifuge (at 14000 rpm for 15 minutes) and if precipitate was present, I separated the solid from the liquid. I analyzed both portions after filtrating them using filters that exclude everything past 16kDa (TvL is abut 60kDa) using an electron-spray ionization mass spectrometer (ESI-MS). ESI-MS measure the mass to charge ratio of particles contained in a sample. I will later post an entry about ESI-MS in my instrumentation blog. Knowing the molecular mass of our original substrate, I was able to identify polymers of that substrate. This is actually very easily done by calculating the separation of adjacent peaks. If that separation equals the molecular mass of the original substrate you found evidence for polymerization and that substrate can now be considered a substrate for TvL. These findings were recently published in two journals and I included the links to this post.
https://www.sciencedirect.com/science/article/pii/S0964830517310910
https://www.sciencedirect.com/science/article/pii/S1878818116304662
Future Research
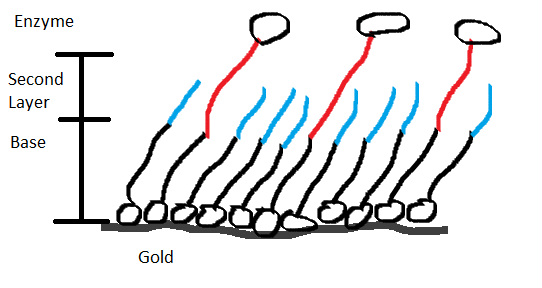
This next semester I will be working on creating a mixed spontaneous assembled monolayer (SAM) which consists of a single layer serving as a foundation that covers the entire surface of a gold substrate and two different organic compounds of different length. The hope is that we will have a gold substrate completely covered by our foundation, with the two different organic substances attached to our foundation. That should give us a SAM that has the longer organic substrate standing out. To the longer organic substrate we will then attach a modified version of TvL to create our water filters.
Thank you for reading! I will elaborate on the progress of this project in the future. If you have questions, please let me know and I will answer them! With my posts I am hoping to motivate people to spend more time reading about science. Many people dislike STEM subjects because they had bad experiences in their early education. I was one of them. But as I got into higher level science, I started to fall in love with research. Hopefully I can get some of you to develop an interest in science.
Cheers!
