You just have to love science! Two separate groups of scientists discovered different ways of converting carbon dioxide into fuel - by using sand or by using bacteria.
Conversion of carbon dioxide to fuel using sand
Researchers from University of Toronto have found a way to convert carbon dioxide emissions into energy-rich fuel through carbon-neutral cycle that uses the second most abundant element in Earth's crust (27.7% of the Earth's crust), and easily available in sand - silicon.
The nanostructured hydrides (or as scientists call it 'hydride-terminated silicon nanocrystals'), with diameter size of about 3.5 nanometres, have a surface area and optical absorption strength ample enough to efficiently gather the near-infrared, ultraviolet and visible wavelengths of sun light, altogether with a powerful chemical-reducing agent on it's surface that selectively and efficiently transforms carbon dioxide into carbon monoxide.
As a result, we acquire energy without harmful emissions.
The study has been published in the article in the journal Nature Communications.
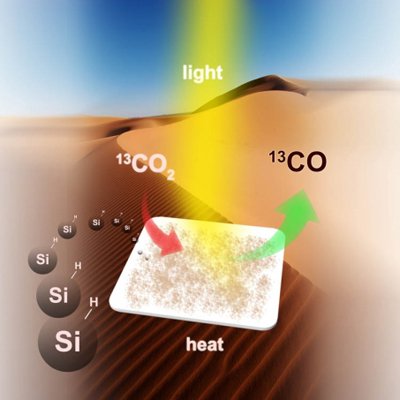
[Process of converting CO2 into fuel using hydride-terminated silicon nanocrystals. Source]
Conversion of carbon dioxide to fuel using bacterium
By using light-driven bacteria, scientists at Utah State University are close to cleanly transforming disastrous carbon dioxide emitted by combustion of fossil fuels into usable fuels. Biochemists produced methane from carbon dioxide in single enzymatic step by using the phototropic bacterium Rhodopseudomonas palustris as a biocatalyst.
According to one of the group leading researchers, USU professor Lance Seefeldt, no other organism can achieve what this bacterium has managed to achieve with this one enzyme. Breaking apart or reducing carbon dioxide molecules requires massive amounts of energy, because carbon dioxide is very stable, so using phototrophs opens a wide range of new possibilities.
Their research has been funded by US Department of Energy Office of Science's Energy Frontier Research Center and the results have been published in official scientific journal of the National Academy of Sciences.

[Biochemists from USU working on the project. Source]
Each year, humanity escalates climate change and global warming by introducing carbon dioxide into the atmosphere (currently about 30 billion tons).
It seems like there is still hope for the planet Earth :-)
-logic
References:
"Heterogeneous reduction of carbon dioxide by hydride-terminated silicon nanocrystals"
http://www.nature.com/ncomms/2016/160823/ncomms12553/full/ncomms12553.html
"U of T scientists solve puzzle of converting CO₂ emissions to fuel"
https://www.utoronto.ca/news/u-t-scientists-emissions-fuel
"Light-driven carbon dioxide reduction to methane by nitrogenase in a photosynthetic bacterium"
http://www.pnas.org/content/early/2016/08/16/1611043113
"USU Biochemists Describe Light-Driven Conversion of CO2 to Fuel"
http://www.usu.edu/today/?id=56068
