Identification of an Unknown Gas Through Application of Spectroscopy

Within an atom, electrons orbit the nucleus in the electron cloud, where they have discrete energy values, or energy levels, that define their location and behavior within the cloud. Electrons can move between energy states, but as energy in a system is conserved, there must be an input of energy to the atom for the electrons to move to a more excited state, and energy must be released when they move to a lower energy state. This energy release is in the form of light emission . Physicists in the late 1800’s observed that that the wavelength spectra emitted from a specific element was consistent. They mathematically derived two main relationships between the element properties and the resulting light spectrum. First, that the wavelength of light emitted by an electron energy level change was dependent on the starting and ending energy level. Second, the relative mass between the electron and the nucleus affected the emitted wavelength. As an element’s emitted light spectra can give information about the elemental properties, spectroscopy has a variety of uses. Here, spectroscopy is used to identify an unknown gas based only on its emitted wavelength.
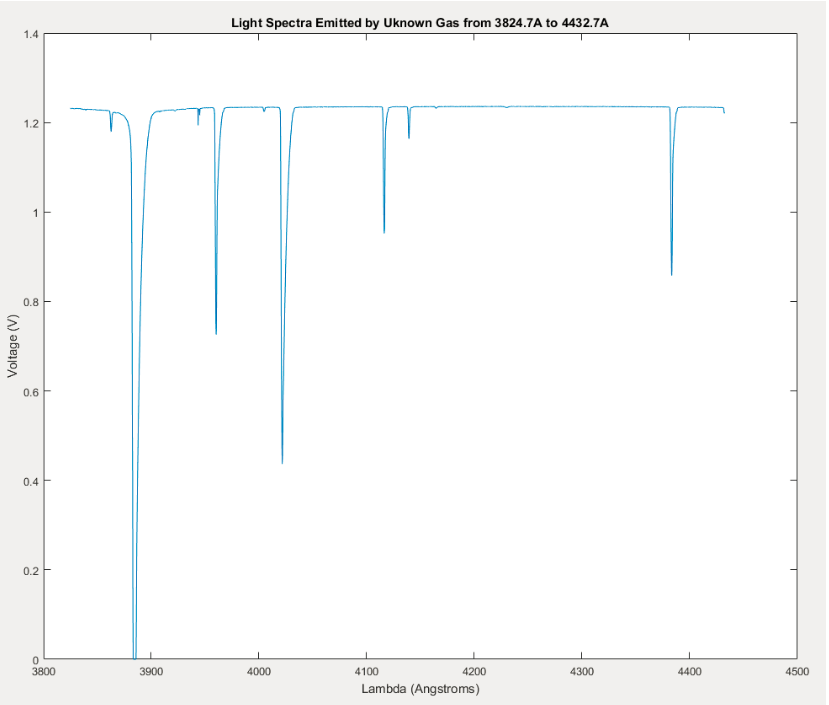
Light Spectra Emitted by Unknown Gas from 3824.7A to 4432.7A. Graph of emission voltage collected from the unknown gas as wavelength changed consistently through time from the 3824.7A and 4432.7A. Voltage minima correspond to peaks in light wavelength intensity.
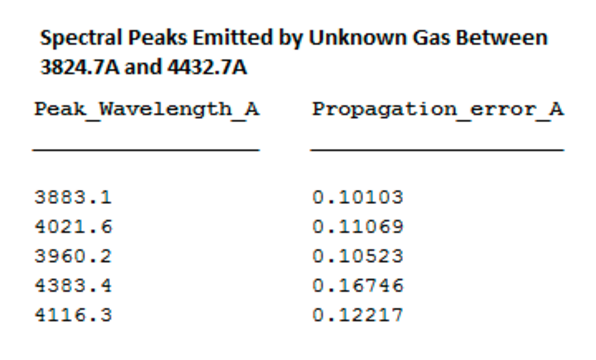
The spectral peak wavelengths of the unknown gas match those of helium, the second most common gas in the universe.
Sources
NIST, Basic Atomic Spectroscopic Data. “Strong Lines of Helium (He). Web. Accessed February 09, 2017. http://physics.nist.gov/PhysRefData/Handbook/Tables/heliumtable2.htm
Taylor, John R. Zafiratos, Chris D. Dubson, Michael A. , 2015. Modern Physics For Scientists and Engineers 2nd Edition. University Science Books. Pages 144-156
