The Degradation of Chlorophyll

Image taken from Source [1]
Basics first:
Why are the leaves of plants green anyway?
The color rises from distinct molecules, which are capable of absorbing elecromagnetic radiation with wavelengths within the visible light (approximately 400-750 nm).
In general such molecules are called pigments.
We then perceive the part of the visible spectrum that has not been absorbed, so the parts that are reflected give the observed so-called complementary color.
During spring and summer, the most abundant pigments in our biosphere are chlorophyll a, chlorophyll b, carotinoide and accessory pigments like photocyanines, from which the first ones are of particular importance for higher plants.
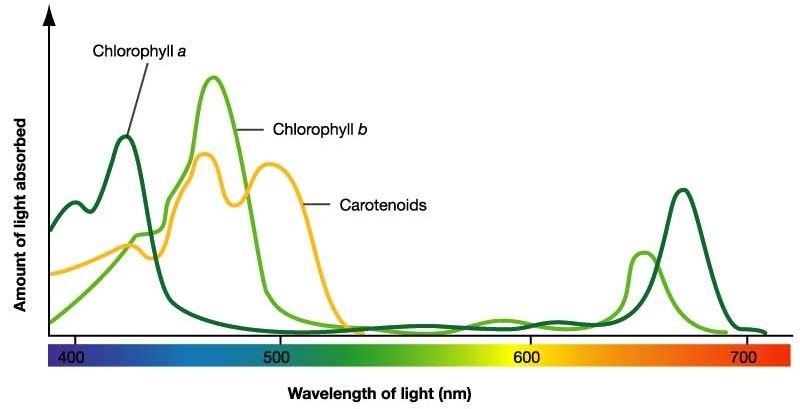
The next image shows the absorption spectra of these major pigments.
As one can easily see, the chlorophylls have in common that they barely absorb in the green area, which means that we perceive this color whenever these pigments dominate in the leaf.
What is the use of those pigments?
Their most important task is to absorb sunlight and to transmit the energy obtained from it to the so called photosystem I and photosystem I. These are sophisticated photosynthetic complexes, which allow photosynthetic organisms to use this energy for the conversion of CO2 into useful carbohydrates.
Why is Chlorophyll degraded at all?
The tree protects itself from the frost by leaf fall. But before they discard the leaves in the fall, the plants get important nutrients, such as nitrogen and minerals (eg from proteins), back from their leaves. In this process chlorophylls are released from the proteins into which they are normally incorporated. In this free form, however, they are phototoxic:
Under light, they can damage the leaf via the formation of celltoxic radical oxygen species.
It must therefore be "detoxified" by degradation.
In addition, some plants are able to reduce the amount of light reaching the chlorophylls by forming red screening pigments (anthocyanins). One may perceive this phenomenon by the bright red coloring of some autumn leaves.
On the chemical steps involved in the degradation of chlorophyll:
While the biosynthesis of chlorophyll has been elucidated over 30 years ago, the biochemistry of degradation has long remained unknown. Prof. Kräutler (University of Innsbruck) is one of the leading scientists concering research on this particular process. A short outline of the degradation and recycling steps that take place in aging leaves and ripening fruits is shown:
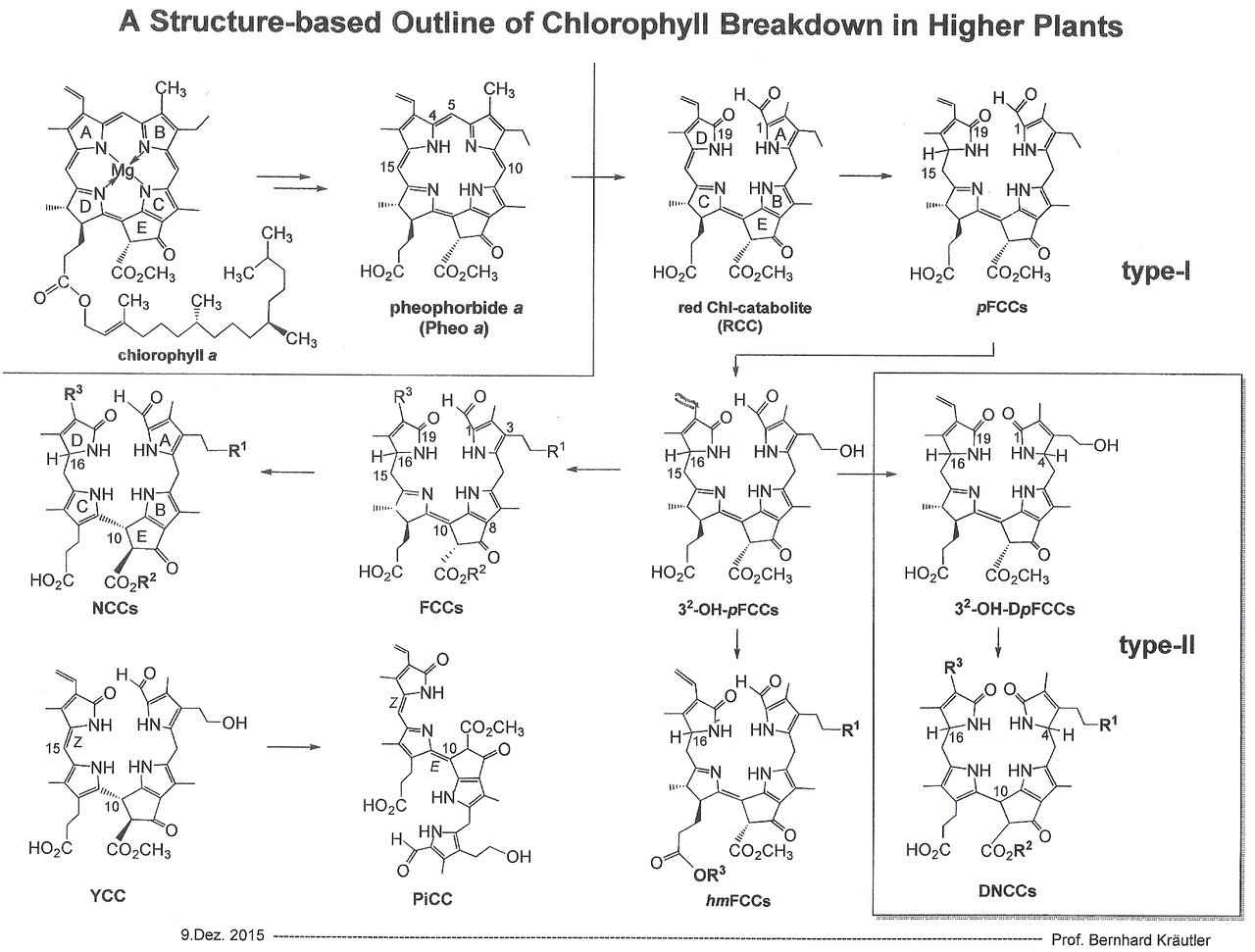
A Handout designed by Prof. Kräutler, University Innsbruck
Chlorophyll's original extended conjugated and cyclic pi-electron system ensures that it has the absorption spectrum shown above with absorption bands in the visible light range.
With the onset of the degradation, there is a drastic change in the absorption properties:
First, a red intermediate is formed, which is produced only in very small quantities and which is quickly transformed into colorless compounds.
This is also the explanation for the wonderful yellow to red colors,
which we are allowed to enjoy in autumn:
Since the usually dominant chlorophyll is degraded, other pigments (carotenoids, assessory pigments) and possibly formed anthocyanins come to the fore and define the color.
Interestingly, during the degradation process of chlorophyll in bananas, a blue fluorescent dye is formed. Ripe bananas therefore glow blue when illuminated with UV light!
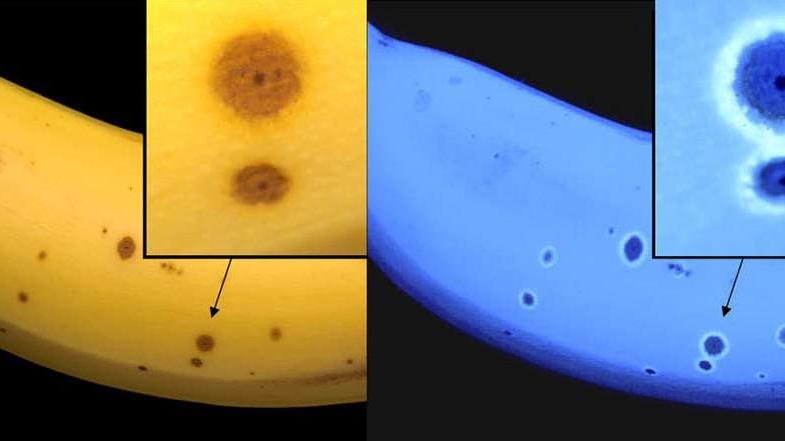
Image taken from Source [2]
Prof. Kräutler assumed that the fluorescent glow may not be a coincidence.
It is known that monkeys see much better in the ultraviolet portion of light than humans. Thus, these animals could easily find ripe bananas in a tangle of leaves, eat them and spread the contained seeds - all as intended by the plant.
Let's end with some short facts concering this topic:
Chlorophyll is the most abundant dye on earth!
(Ref. 2)It has been estimated that about 1 billion tonnes of chlorophyll are degraded each year!
(Ref. 5)Over 75% of this conversion is caused by organisms living in the water (algae, cyanobacteria,...)!
(Ref. 5)
I hope you have enjoyed this article.
If you have any remaining questions do not hesitate to ask.
For more like this follow me!
Best,
mountain.phil28
References:
[1] A Chemistry World Article
[2] Two science.ORFArticles: Article a & Article b
[3] An Article in Biologie unserer Zeit
[4] An Archive report by the University of Innsbruck
[5] An Article in Naturwissenschaften , Volume 76 (10) – Oct 1, 1989 by Wolfhart Rüdiger