Everyone knows that oxygen is required to live.
But, why is that the case? What is it about oxygen that is essential for life?
To be able to harness the of energy from nutrients is essential for all life. Each cell must generate energy in order to fuel the chemical reactions that occur within it. These biochemical processes then come together to allow the human body to grow, repair and function in a normal way. This process is known as aerobic respiration, and as the name suggests oxygen is a necessity.
The majority of this energy is generated within a specialised organelle, known as the mitochondria. It is important to note that although the words to generate are used, energy is not created by mitochondria, as this would break the first law of thermodynamics. The intended meaning for these terms is that energy from molecules such as glucose is used to create small packages of energy that can be utilised throughout cells. This occurs in the form of ATP.
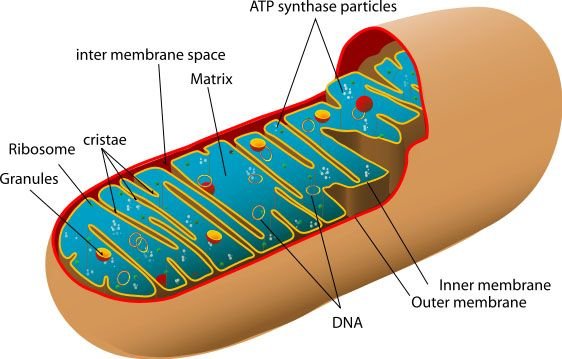
figure 1. A mitochondrion
Why use ATP?
Adenosine triphosphate (or ATP), is a very convenient way to package the energy created from respiration. ATP is simply an adenine base attached to a ribose sugar, with three phosphate groups added on the end.
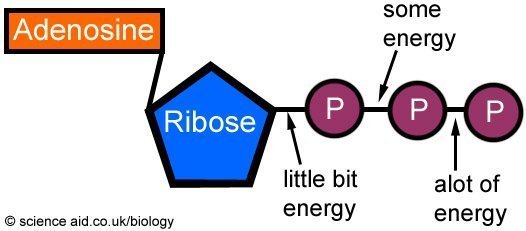
figure 2. ATP
ATP is useful as an energy currency because each of the phosphates can be removed from the molecule in a hydrolysis reaction giving off an appropriate packet of energy. The addition of water to ATP will form ADP (adenosine diphosphate), a free phosphate (Pi) and energy. This energy is used to fuel chemical reactions throughout the body. ADP can also be hydrolysed to AMP (adenosine monophosphate) to release more energy.
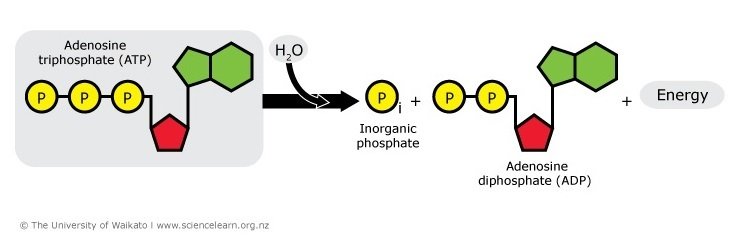
figure 3. ATP to ADP
To understand the entire process of respiration, I will be using the example of glucose to determine how a molecule can be used to generate ATP, which will be used to fuel chemical reactions.
How does glucose become ATP?
It isn’t quite as straightforward as breaking down glucose to provide energy for a reaction that forms ATP. It is a multi-step process, involving multiple enzymes and cofactors. The first stage of this process is called glycolysis, in which glucose is broken down.
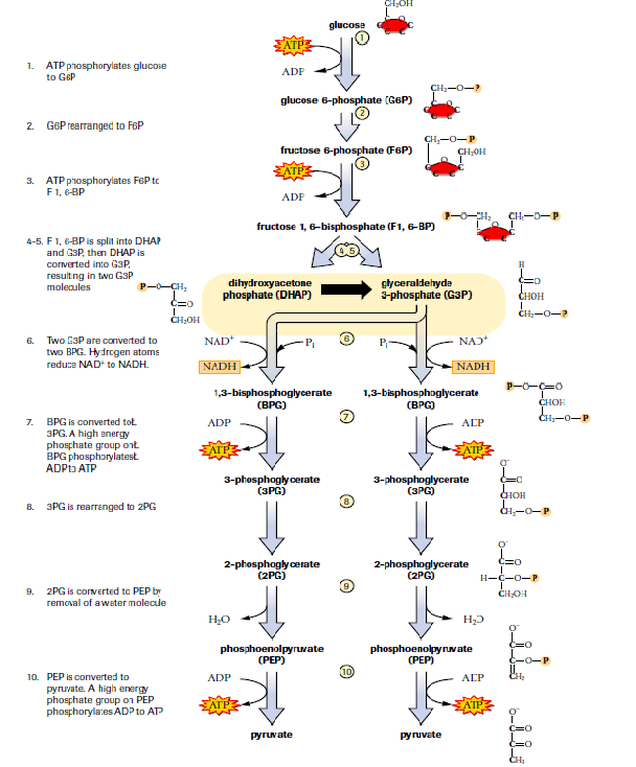
figure 4. Glycolysis
This process results in the production of 2 pyruvate molecules per glucose. It also has a net production of 2 ATP per glucose and 2 NADH. It is interesting that the first stages of this process actually consumes 2 ATP (the molecule we are attempting to produce). In this case some energy needs to be used up to rearrange and split the glucose, which ultimately leads to far more energy being created down the line. The steps of glycolysis do not occur in the mitochondria, but just in the cytoplasm of the cell. Perhaps the most important molecule produced in glycolysis is NADH. This is a coenzyme, which is used to help enzymes undertake oxidation and reduction reactions. The reduced form being NADH and the oxidised form being NAD+.
Pyruvate is then converted to acetyl-CoA in a reaction known as the link reaction (so named because it links glycolysis and the Krebs cycle).
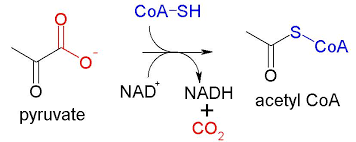
figure 5. The link reaction
It is worth nothing that another NADH molecule is produced, along with CO2.
Acetyl-CoA is then able to enter the Krebs cycle, which occurs in the matrix of mitochondria (see figure 1). The Krebs cycle can also be known as the citric acid or tricarboxylic acid cycle. In full detail, this cycle is extremely complex, but I will just highlight the important information.
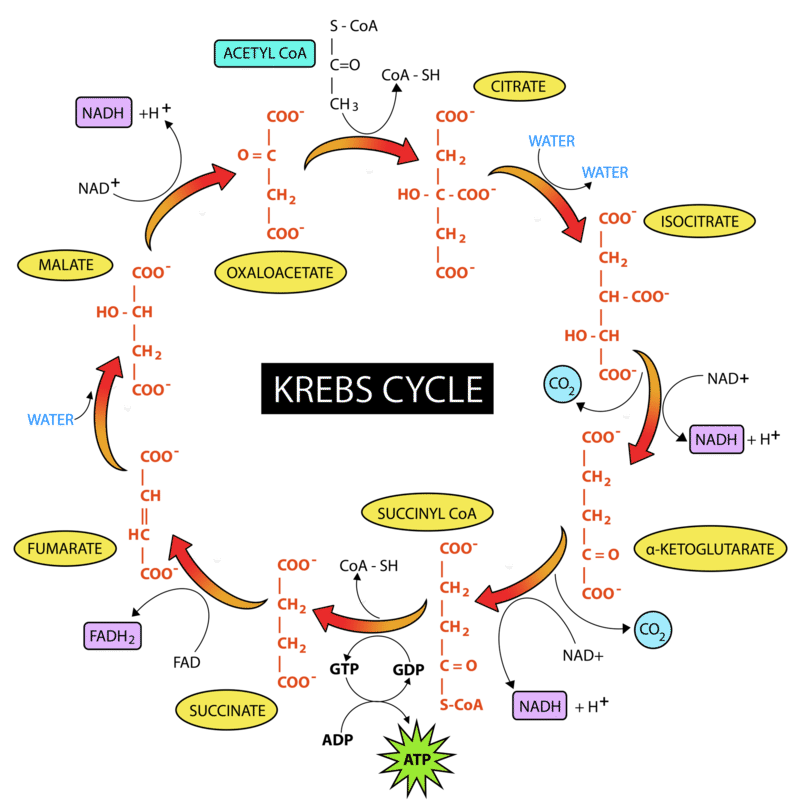
figure 6. The Krebs cycle
It is important to note that for every turn of the cycle 3 NADH, 1 FADH2 and 2 CO2 is formed. This means that double these quantities are made for each glucose molecule entering glycolysis, since each glucose yields two pyruvate molecules, and therefore two acetyl-CoA molecules.
It may not have escaped your attention that so far, not much ATP has been made. This is because the majority of ATP is formed from the final step of aerobic respiration, known as oxidative phosphorylation.
How does oxidative phosphorylation yield so much ATP?
There are two membranes surrounding mitochondria, an inner and an outer membrane (figure 1). ATP is made by joining an ADP molecule and Pi molecule together, which occurs in an enzyme known as ATP synthase.
Hydrogen ions (H+) move through ATP synthase, down a concentration gradient, from between the two mitochondrial membranes into the matrix, and this provides the energy to form ATP from ADP.
How is the hydrogen ion gradient set up?
This is where the cofactors finally become useful. NADH and FADH2 are oxidised to produce H+ and electrons (e-). The electrons then provide the energy for various complexes to pump H+ from the matrix to the intermembrane space of the mitochondria, building up a concentration gradient. The inner mitochondrial matrix is impermeable to H+ ions, the only way for the concentration gradient to be dissipated is through ATP synthase, thus generating ATP. This is appropriately called the electron transport chain. There are 4 complexes within the chain, only 3 of which pump H+ across the membrane. Complex 1 pumps 4 H+, complex 2 pumps no ions, complex 3 pumps 4 H+ and complex 4 pumps 2 H+ ions. Oxygen is required in this process as a final electron acceptor, without it, the electron transport chain will grind to a halt and very little ATP will be produced. With oxygen present, H+ ions are continually cycled through ATP synthase, constantly forming more ATP.
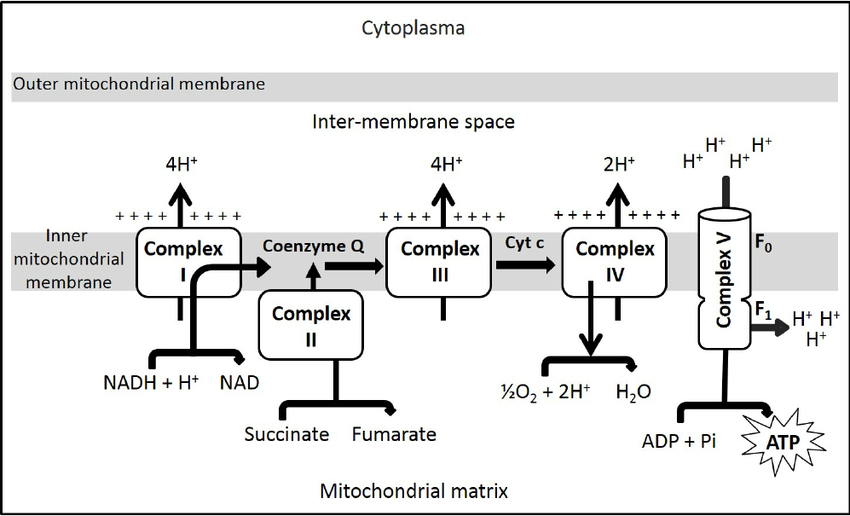
figure 7. The electron transport chain
In figure 7, complex 5 is referring to ATP synthase.
Finally, through the use of NADH and FADH2, glucose has generated ATP, in a rather convoluted and horribly complicated process. Each glucose molecule can produce 32 molecules of ATP through this indirect pathways. It is astonishing that such a seemingly simple chemical reaction can have such complicated biochemical processes behind it.

figure 8. Aerobic respiration formula
Each of the descriptions I have given are mere summaries of the exact events that take place. However, I have aimed to give an overview of aerobic respiration, in order for readers to be able to understand how ATP is generated in the body.
References:
First law of thermodynamics
ATP
Glycolysis
Krebs cycle
Number of ATP produced per glucose
Oxidative phosphorylation
