Hello everyone,
I previously wrote about common types of radiation. Click here to see that post.
This writeup is going to be about more uncommon ionizing radiation variants. Most of these can be picked up by hobbyist equipment but aren't particularly important for a hobbyist. That being said, they are still interesting: Hence why I am writing this post. I hope you learn something new from this.
Once again, this is only covering ionizing radiation (radiation that can ionize atoms and break up molecules). Please see my previous post, linked above, for a brief overview of this.
Neutron Radiation
Free neutron radiation is particularly interesting. As I'm sure you already know, neutrons are essentially slightly more massive protons with no net electric charge. This means that they can interact with other particles via gravity, the weak force, and the strong force, but NOT the electromagnetic force. This makes them notoriously hard to shield, since for many materials, neutrons are completely unaffected by moderately thin shielding.
Other than protons and antiprotons/neutrons, neutrons are the most stable unstable hadron. In stable atoms, neutrons are stable and do not decay. However, neutron radiation consists of free neutrons, which are unstable. Free neutrons decay with a mean lifetime of around 14 minutes via beta decay, releasing a free electron, electron antineutrino, and proton. In practice, this rarely happens, because most free neutrons will be absorbed before they have a chance to decay, as I will elaborate on below. You can read more about neutron decay here.
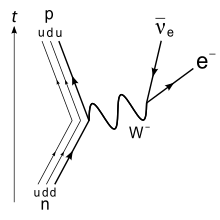
Neutron Decay: Image credit
Radiation damage caused by free neutrons is almost always not due to decay but due to either elastic collisions or neutron capture. In an elastic collision, the free neutron strikes a nucleus and recoils off with an energy based on the mass of the nucleus. This then produces a free ion (the nucleus the neutron originally hit) that is then itself ionizing radiation. In neutron capture, a free neutron is absorbed via the strong force into the target nucleus, turning the nucleus into a different isotope of the same element. This can result in the newly formed nucleus decaying, fissioning (this is what happens in nuclear bombs), or letting off a gamma ray and staying as the final isotope. This isotope can also be unstable and might decay on its own sometime later.
An interesting quality of free neutrons is that their absorption cross sections (probabilities) increases as the neutron's kinetic energy decreases. Very slow neutrons are called thermal neutrons, and many nuclei have very high thermal neutron absorption cross sections. You can explore some of the data on this at this ENDF database.
To protect against neutrons, you'll want to absorb them. This can be done by using a material with a very high neutron absorption cross section such as cadmium (See an example of this here) and then shielding secondary radiation/decays. To help the process, proton-rich materials can be used to slow down the neutron radiation before it comes to the shielding material, to increase the cross-section. Materials like water or many polymers have lots of hydrogen atoms in them and work for this. The reason that protons slow down neutrons effectively is because an elastic collision of a fast neutron hitting a stationary proton will transfer almost all of its kinetic energy to the proton and leave the neutron moving quite slowly.
You can also use this principle to detect neutrons. For example, wrapping a geiger counter tube in cadmium or some other good neutron absorbing material will make a crude neutron detectors, but it would only work with high neutron fluxes. A better option is a He-3 detector surrounded by proton-rich material, which will slow down neutrons to be absorbed by Helium-3 inside the tube. I wrote about this a little in my radiation detector post: Check it out if you're interested.
Neutrons are part of normal background radiation, produced by cosmic ray collisions in the atmosphere. They are also produced in fusion reactions: Deuterium-deuterium (D-D) fusion, in particular, produces a lot of neutrons. Fission of heavy elements like uranium also produces neutrons. Finally, certain elements can react (basically fuse) with alpha radiation to produce neutrons, such as beryllium. An alpha source pressed up against a beryllium bar will produce a little bit of neutron radiation. I don't recommend you try this, since any legal alpha source will produce a pitiful amount of neutrons, and beryllium is highly toxic.
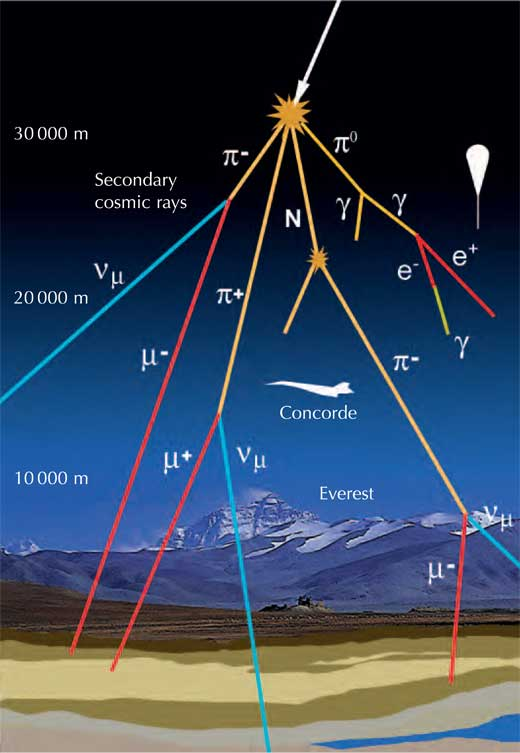
Cosmic Rays
Cosmic rays are a mix of electrons and nuclei (mostly protons) moving with absurdly high kinetic energy. They strike the Earth at a pretty constant rate and originate outside the solar system. Cosmic rays don't often reach the ground due to collisions with gas atoms in the atmosphere, and are more hazardous in their direct form in space. Cosmic ray energies are truly massive for individual particles: One of the highest energy cosmic rays ever detected, the Oh-My-God-Particle, had a kinetic energy of about 50 Joules. If that doesn't shock you, think of it this way: That single tiny nucleus was moving with the same kinetic energy as an average-weight person walking at a slow pace.
Cosmic rays can also produce tons of other subatomic particles when they hit and react with atoms in the atmosphere. Some of these include pions, which are unstable mesons (quark-antiquark bound pairs) that decay very quickly, producing the muon radiation we see on the ground (see my last post for muon radiation details). Pions are pretty interesting, and I'd highly recommend reading the wikipedia article on them if you haven't already.
In general, you can't really shield cosmic rays, and there aren't that many of them. On the off chance that a cosmic ray makes it through the thick atmosphere of Earth, it can be detected on any radiation detector.
Proton Radiation
Proton radiation is made up of regular hydrogen nuclei moving at high speeds. It can be produced in radioactive decay, but typically only occurs in very short lived isotope decays, and as such is pretty rare for a hobbyist to observe it. Proton ionizing radiation can also be produced by the elastic neutron collisions mentioned above.
A proton: Image credit
There's not a whole lot to say here. Protons, having 1/4 of the mass and 1/2 the charge as alpha (He-4) particles, are harder to shield than alpha radiation at comparable energies. Having a higher mass, they will be easier to shield than comparable energy beta radiation but will do more biological damage to cells.
Proton radiation is much more common in space. The Van Allen radiation belts (produced by the Earth's magnetic field) are full of very high energy proton radiation that can be damaging to spacecraft passing through them. Many of these protons are thought to come from neutrons produced in cosmic ray collisions that traveled away from Earth before decaying into protons and subsequently being captured in the Earth's magnetic field.
As a hobbyist experimenting with radiation, you won't see proton radiation too often. That being said, many geiger counters and photodiode detectors should be able to pick fast protons, especially at higher energies.
Shielding protons just requires slightly thicker alpha radiation shielding. Their high mass compared to electrons means that very little braking radiation will be produced (the acceleration will be less), so secondary XRays are not very important here.
Conclusion
As mentioned before, with the possible exception of cosmic rays these radiation types aren't very common for someone just making radiation detectors and trying them out on the ground. That being said, I still think it's interesting/important to understand what they are and how they work.
Let me know if I missed anything. I hope you were able to learn something from this post. If you have any questions, comment below and I'll do my best to answer.
Thanks for reading!
Tips/Upvotes appreciated!
NAV: NZLuonFWZTRL8mYQ41SwaP7qopSTbgDQ72
BTC: 1MV7pvR9PGzYBMbSpAyRQbMBonJxZyBnrT
VTC: ViuLmSCuyQXLTJKZphKPwmTvFfvjc88Woz