5.0 BCL-2 an anti-apoptotic protein?
The anti-apoptotic BCL-2 protein is chiefly found embedded in the OMM and the ER. It is regulated by transcriptional factors, and posttranslational modification. BCL-2 exerts its anti-apoptotic effects by preventing homodimerisation of BAX and BAK at the OMM by interacting with the pro-apoptotic BH-3-only proteins. It also interacts directly with BAX and BAK inactivating them.
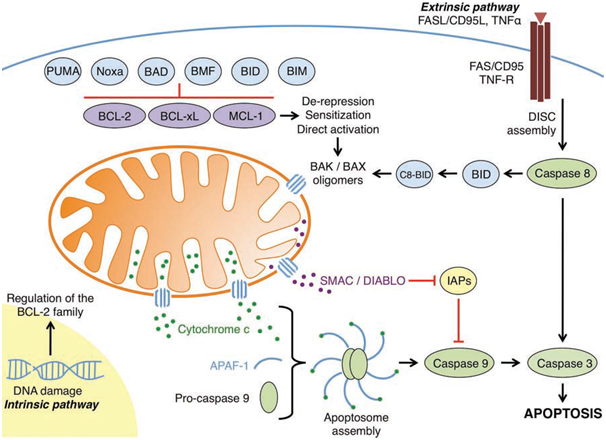
Figure 1: An overview of the intrinsic and extrinsic apoptotic pathways
(Elkholi et al., 2011).
BCL-2 protects a cell from a wide variety of stresses and so regulating the expression of this protein is important. The BCL-2 gene contains three exons and two major promoters, P1 and P2. The major transcriptional promoter P1 contains the Sp-1-binding site and cyclic AMP response element (CRE). These sites are controlled by transcriptional factors such as c-Jun and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). ROS have been shown to downregulate expression of BCL-2. ROS decreases expression of BCL-2 by promoting binding of CRE-binding protein and/or NF-κB to CRE. This ROS/NFκB pathway has been shown to be regulated by sigma-1 receptors that promote cellular survival (Meunier and Hayashi, 2009). Under certain conditions ROS can promote degradation of BCL-2. Regulating the expression of BCL-2 is important as it dictates the BCL-2:BAXheterodimer ratio, and controls whether the cell undergoes apoptosis or not.
BCL-2 has been show to be ubiquitinated at lysine 17 and degraded via interaction with cytostolic inhibitor of Nrf2 (INrf2) at its BH2 domain (Niture and Jaiswal, 2011). NF-E2-related factor 2 (Nrf2) is a regulator of cytoprotective gene expression that is also ubiquitinated and degraded via interaction with INrf2. Antioxidants can disrupt INrf2:BCL2 resulting in stabilised BCL-2. The pro-apoptotic protein INrf2 downregulates BCL-2 leading to a lower threshold of stimuli required to induce apoptosis due to a decrease in BCL-2:BAX heterodimers. This regulation of BCL-2 functions under similar mechanisms as reduced transcription.
The BH-4 domain of BCL-2 has been shown to be integral to its anti-apoptotic function. One such function is to interact via its BH4 with Raf-1 targeting it to the mitochondria (Wang et al, 1996). Raf-1 that has been targeted to the mitochondria interacts with and phosphorylates BAD, inhibiting its proapoptotic function, and thereby promoting resistance to apoptosis. This function of BCL-2 shows one if its many mechanisms in regulating apoptosis dependently of directly binding to any BH3-only proteins or BAX.
The anti-apoptotic protein BCL-2 has a complicated role in regulating apoptosis as it can be converted into a pro-apoptotic protein. BCL-2 is structurally homologous to BAX. Oligomerisation of BCL-2 can be induced by the direct activator tBID (Peng et al., 2009). Although tBID changes BCL-2 into an active BAX like conformation, this process does not drive apoptosis. The proteolipid pores formed in the OMM by BCL-2 via tBID induction are not large enough to allow cytochrome c release. This pore forming function of BCL-2 promotes cell survival by preventing association between tBID and BAX.
The direct activator tBID fails to convert BCL-2 into a proapoptotic protein, but interaction with the direct activator BIM has been shown to convert BCL-2 into a proapoptotic BAX-like molecule (Zhao et al., 2011). This conversion of BCL-2 into a proapoptotic protein only occurs during elevated expression of BIM. This is due to the size of the BCL-2 pore being dependent on BCL-2 and BIM levels. BIM migrates to the OMM and interacts with, causing conformational change and oligomerisation of BCL-2 resulting in caspase release. Whether this occurs in vivo or not is not known, as BIM is predominantly sequestered in the cytosol by microtubules and so unable to interact with BCL-2. Also BIM preferentially interacts with BAX and BAK, indicating that BCL-2 interaction and oligomerisation may only occur when BAX and BAK are not available.
BCL-2 can also be converted into a pro-apoptotic protein by mechanisms that occur downstream of BAX or BAK-mediated MOMP. BCL-2 can be cleaved by caspase-3 at its BCL-2 loop which links the BH3 and BH4 domains. This converts it into a pro-apoptotic BAX-like molecule. This function of BCL-2 helps to increase the efficacy of apoptosis after the caspase cascade occurs by increasing MOMP and also preventing BCL-2 from inhibiting any pro-apoptotic proteins.
The nuclear receptor protein Nur 77 has been shown to convert BCL-2 into a pro-apoptotic protein by binding with its BCL-2 loop and exposing its BH3 domain (Kolluri et al., 2008: Thompson and Winoto, 2008). This pro-apoptotic BCL-2 does not form pores but instead interacts via its BH3 domain to activate BAX and BAK. This pro-apoptotic mechanism of BCL-2 is BAX and/or BAK-dependent. Nur77 only translocates to the cytosol during pro-apoptotic stimuli and nuclear pore destabilisation, highlighting this mechanism of BCL-2 as occurring downstream of BAX/BAK-mediated MOMP. This function of BCL-2 may work in synergy with the caspase cleavage of BCL-2, to prevent BCL-2 from inhibiting apoptosis past the point of no return, and to increase the speed and efficacy of this process. The protein p53 can also bind the BCL-2 loop which also results in BCL-2 acting like a BH3-only protein. Interactions with the BCL-2 loop regulate the function of the BCL-2 protein in many different ways.
BCL-2 predominately exerts its anti-apoptotic effects by interacting with BAX or by sequestering the direct activators of BAX. The non-apoptotic oligomerisation of BCL-2 inhibits oligomerisation of BAX. This and the similar shared structure between BCL-2 and BAX suggest that BCL-2 is a defective BAX molecule. This is concordant with the mechanisms in which BCL-2 regulates apoptosis. The anti-apoptotic protein BCL-xL also shares structural homology with BCL-2 and BAX, and like BCL-2 can also be converted into a pro-apoptotic protein. This highlights the complex nature of the BCL-2 family and shows that each protein can function in a plethora of ways.
Part 1
Part 1.1-2
part 2
part 3
part 4
part 6
@RiskDebonair
Adventure Capitalist of the Future
