By Roentgen or X-rays, we usually mean electromagnetic radiations has a wavelength shorter than that of ultravoilet light though, there is no sharp boundry. The range is usually considered to be 0.01 nm to 10nm, which corresponds to quantum energies of 100 eV to 100 keV.
credit
X-rays are produced if heavier atom are bombarded by electrons which have been accelerated through thousands of volts. They were first observed by Wilhelm K-Roentgen in 1895. Thus X-rays are also known as Roentgen rays.
Production
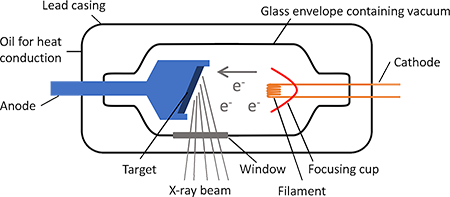
Electrons released from the heated cathode by thermoionic emission are accelerated toward the anode(metal) by a large potential difference. It is found that at sufficiently high potentials (several thousands) a very penetrating radiation is emitted from the surface of the anode. As the electrons collide and interact with the atoms on the anode target, a great amount of energy is produced. 1% of this energy is in the form of x-radiation. 99% appears as heat and must be removed from the anode.These rays are of the same nature as light or any other electromagnetic wave.
credit
The X-rays are detected by photographic plates, film, counting tubes, or more recently, by semiconductor detectors. In 1913 W.H. Bragg discovered the phenomena of X-ray diffraction by crystals. This technique permitted the precise measurement of the wavelength of X-rays and thus became the basis for a study of X-rays spectrum.
Spectral analysis of X-rays shows that:
1) there is always a continous spectrum i.e the bremsstahlung
2) under certain conditions, there is in addition a line spectrum, the characteristic spectrum.
Continous spectrum or Bremsstahlung
Bremsstrahlung (German pronunciation: [ˈbʁɛmsˌʃtʁaːlʊŋ] (About this sound listen), from bremsen "to brake" and Strahlung "radiation"; i.e., "braking radiation" or "deceleration radiation")
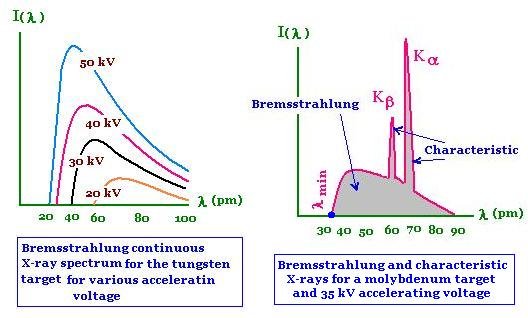
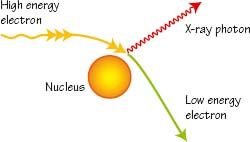
Ordinarily, a continous of frequencies of X-rays is emitted, but the maximum frequency or minimum wavelength was observed to be always directly proportional to the accelerating voltage between the electrodes. Furthermore, this maximum frequency was found to be very nearly independent of the material of which the electrodes were made. These observations can be understood on the basis of quantum theory. The bremsstrahlung spectrum is a result of the fact that when electron pass close to the atomic nuclei, they are deflected and slowed down.Since the rate of deceleration is so large, the emitted radiation correspond to short wavelength. In the case when the electrons lose all their kinetic energy in the first collision, the entire kinetic energy appears as X-ray photon of energy hfmax. The λmin in below figure corresponds to fmax. Other electrons do not loose all their energy in the first collision. They may suffer a number of collission before coming to rest. This will give rise to photons of smaller energy or X-rays of longer wavelength. Thus the continous spectrum is obtained due to deceleration of impacting electrons.
credit
A positive or negatively accelerated charge will according to classical electrodynamics, emit electromagnetic radiation. This is a continous x-rays bremmsstrahlung. Interms of quantum theory, this can be understood as follows: for each braking incident, a quantum of light hf = Eo - E is emitted. However, since the beginning and end states are not quantized. The process is represented as:
credit
Atom + e-(fast) →→→ Atom + e-(slow)+ hf (X-ray)
Charactetistic Spectrum or Line spectrum
The spectrum of X-rays consist of a continous spectrum upon which is superposed a line spectrum. The distribution of energy in the continous spectrum depends only upon the potential difference across the X-ray tube while the line spectrum is the characteristic of the target. These are called characteristic spectraband were investigated by Moseley in 1913 by making each element in turn the target in an X-ray tube. Thirty nine elements extending from aluminium to gold were examined in this way. The X-rays were analyzed with bragg crystal spectrometer. Most elements showed two group of lines, one generally less than about 0.1 nm called the K-series and another greater than 1nm and called the L series. The wavelength of the L-series were roughly ten times as great as those of the K-series. For elements whise atomic number exceded, further series appeared which were called the M and N-series.
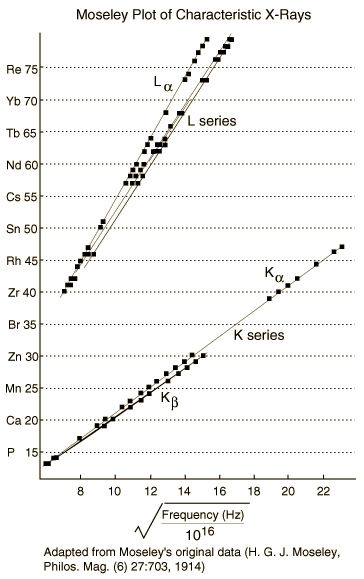
credit
The characteristic X-ray spectra can be explained from the principle of inner shell transition. The electrons of an atom are ordered according to their arrangement in shells about the nucleus. Each shell has a certain maximum number of electrons. There is a rule that the number of electrons in the nth orbit is equal to 2n2. According to this rule, there cannot be more than 2,8,18,32..... electrons in the orbits or shells for n=1,2,3,4.... These orbits or shells are called K,L,M,N.... for n=1,2,3,4.....
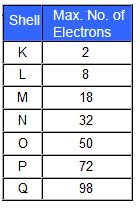
credit
When highly energetic incident photon electron knock an electron from the K-shell, there occurs a vacancy in that shell. This vacant space in the K-shell is filled by an electron from the L-shell giving up energy in the form of an X-ray. This radiation, which is characteristic of the target material, is labeled then Kα line. The electron in the M-shell that fills a vacancy in the K-shell gives up the energy as an X-ray called the Kβ.
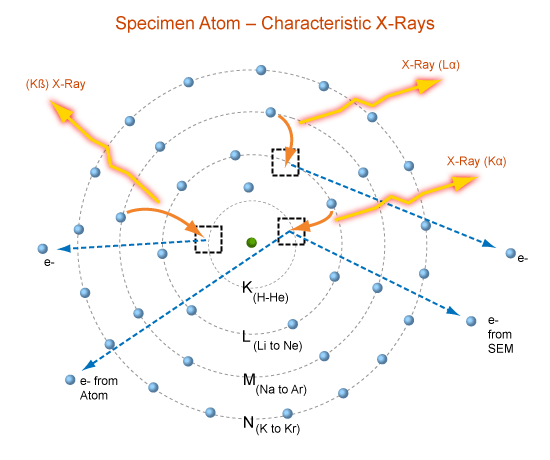
credit
These transition from the shells L,M,N and so on to the K-shell give rise to a series of lines Kα, Kβ, Kɣ and so on called the K-series. When incident electrons dislodge electrons from the L-shell, these are filled by electrons from the remaining M,N,O shells etc. These transition give rise the L-series, the first line of which is L α. The nomenclature of these transition is illustrated in above figure.Upon closer observation each of the characteristic X-ray line is found to be composed of a number of closely spaced lines called X-rays fine structure.
Uses of X-rays
X-rays have many practical applications in medicine and industry. Because X-rays can penetrate several centimeters in to a solid matter, so they can be used to visualize the interiors of the materials opaque to orsinary light, such as fractured bones or defects in structural steel. The object to be visualized is placed between an X-ray source and a large sheet of photographic film; the darkening of the film is proportional to the radiation exposure. A crack or air bubble allows greater amount of X-rays to pass. This appears as a dark area on the photographic film. Shadow of bones appears lighter than the surrounding flesh. It is due to the fact that bones contains greater proportions of elements with high atomic number and so they absorb greater amount of incident X-rays tehn flesh. In flesh, light elements like carbon, hydrogen and oxygen predominate. These elements allow greater amount of incident X-rays to pass through them.
CAT Scanner
In the recent past, several vastly improved X-ray techniques have been developed. One widely used system is computerized axial tomography; the corresponding instrumentbis called CAT-Scanner. The X-ray source produces a thin fan-shaped beam thatbis detected on the opposite side of the subject by an array of several hundred detectors in a line. Each detector measures absorption of X-ray along a thin line through the subject. The entire apparatus is rotated around the subject in the plane of the beam during a few seconds. The changing reactions of the detectors are recorded digitally; a computer processes this information and reconstructs a picture of different densities over an entire cross section of the subject. Density differences of the order of one percent can be detected withCAT-Scans. Tumors and other anomalies much too small to be seen with older techniques can be detected.
Biological Effect of X-rays
x-rays cause damage to living tissues. As X-rays photons are absorbed in tissues, they break molecular bonds and create highly reactive free radicals ( such as H and OH), which in turn can disturb the molecular structure of the proteins and especially the genetic material.
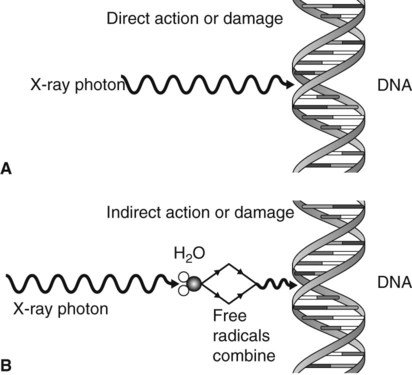
credit
Young and rapidly growing cells are particular susceptible; hence X-rays are useful for selective destruction of cancer cells. On the other hand a cell may be damage by radiation but survive, continue dividing and produce generation of defective cells. Thus X-rays can cause cancer. Even when the organismmshow no apparent damage, excessive radiation exposure can cause changes in their reproductive system that will affect the organism's offspring.
That is all for now. Upvotes , comment & Resteem would be appreciated. Have a nice day!
