Confused about the title? I bet you are. Here and there I hear people say glass is a liquid, then again I hear it’s a solid. Isn’t it obvious? It’s hard and doesn’t move. While you can hold a glass you can’t hold water. So why are there people claiming glass is a liquid? Their answer is simple: look at the glasses of churches. It’s thicker at the bottom than at the top. This is the evidence glass is liquid and moves very slow. We will find out and see whether it is a liquid or a solid.
What is glass and how is it manufactured?
The main chemical compound of glass is lime (10%), soda (13%) and silicon dioxide (70%). Ever heard of it? Sand mostly consists of silicon dioxide, means you could get some sand and make your own glass, theoretically. The sand has be free from any impurities. Iron particles would color the glass green for instance. The melting point of quartz sand is approximately 1700°C to 1800°C. Because such high temperatures are not used in industrial productions, soda (sodium carbonate) is added. This is done to reduce the melting point to approximately 1400°C. Depending on the use of your glass you can add different chemicals to adjust its properties. Heating the sand at around 1400°C it melts and looks like lava. Here is a picture of melted sand. This is basically our glass. A liquid?

Fig 1. Melted sand in an oven Credits
After the melting comes the next step: the glassblowing. This is the place where the glass gets its shape. This is similar to blowing soap bubbles. Molten glass is blown into a bubble. The tool is a blowpipe. These are usually very long pipes to protect the glass blower from the heat and allow him to put air into the bubble. While blowing he forms and cuts the glass into its final shape. After the blowing the glass has to cool down.

Fig 2. Glass pipe in use Credits
When a liquid cools down it becomes a solid. Let’s take water for instance. When we cool water it freezes and becomes ice. The inner structure changes and molecules arrange in a crystalline structure. glass however doesn’t turn its inner structure into a complete arranged grid, glass is only partly a crystalline structure. Solids have a fixed structure of their atoms. As the following picture shows silicon dioxide in nature has a crystalline structure. In glass however it’s structure is not arranged. Such materials are called amorphous solids. They have the characteristics of solids and liquids. Glass is often described as a frozen supercooled liquid.
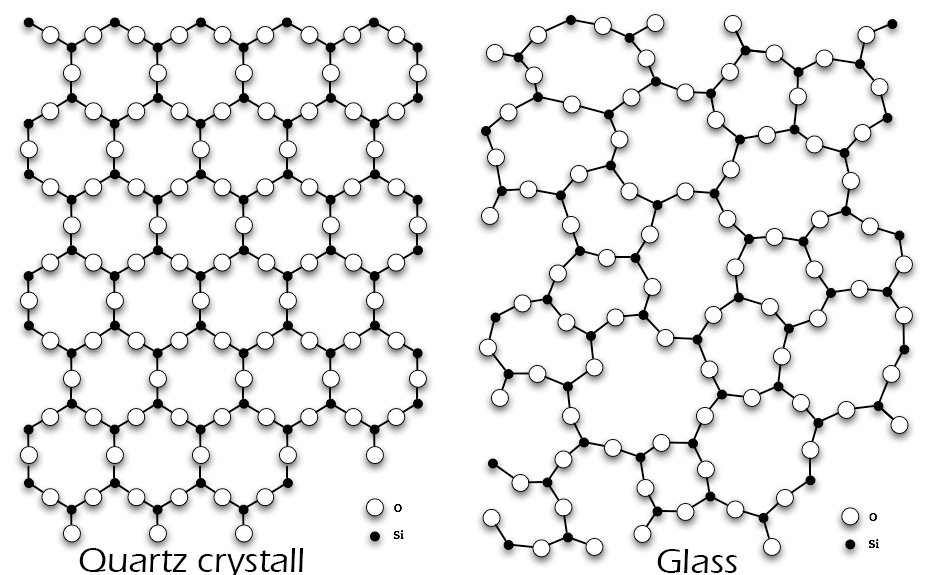
Fig 3. Left: molecular structure of silicon dioxide in nature in crystals. Right: in glass Credits
So why are the glasses in churches thicker at the bottom? Simply because of the way they were manufactured. While the inner structure of glass might change and the atoms move, it would take more time than the universe already exists until the glass becomes thicker at the bottom. Glass is whether a liquid nor a solid. It’s an amorphous solid.
