Welcome back! It’s been a while but here we are with another episode of Myth or Fact! Straight from the laboratory I have something to tell you. So I was sitting with a coworker as he started to tell me his neighbor accidentally swallowed some hydrochloric acid. I was shocked, how could this happen? However the story turned good and nothing serious happened at all. The neighbor drank some base because he heard that the reaction between acid and base will neutralise the dangers. Sounds pretty much like a fairytale right? Let’s investigate!
pH and pOH!
In order to understand what an acid and base is, we need to know the differences between both. To determine the acidity and basicity of a solution, we will define the pH/pOH value. The pH value gives us the acidity of a solution. It’s defined by the negative decadic logarithm of the hydrogen ions activity.

Sounds complicated? It’s not! When you put acid into water it will dissociate (break apart) and release hydrogen ions (H+). These ions are measured then. The more we have of them, the more acid the solution. The pH scale goes from 0 to 14 with 0 being the most acid and 14 the most basic. Water has a pH value of 7 which makes it neutral.
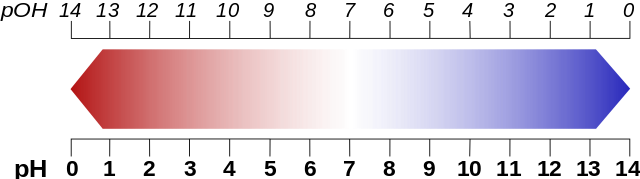
Fig. 2 pH scale. Source
The pOH value is similar to the pH value. It describes the basicity of a solution and is defined by the negative decadic logarithm of the hydroxide ions activity. You can say that when your solution has a pH value of 2 its pOH value is 12. Why? Because pH + pOH = 14! Back to the lucky neighbor who drank hydrochloric acid.
Acids and Basis
Hydrochloric acid, also known as HCl, is a very strong acid. You don’t want this to get on your skin, trust me. In water it turns into H3O+ and Cl-. Where does H30+ come from? Well as I said acids release hydrogen ions (H+), these put together with water molecules (H20) and form H30+ ions. If want to know more about acids I really recommend you to get a chemistry book or contact me in the Steemit.chat, but as for know this is all what we need to know.
Fig. 3 Hazard Source
A common base is sodium hydroxide, NaOH. In water it dissociates into Na+ and OH-, both ions as you can see on the positive and negative charge.
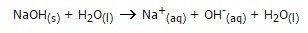
Both are dangerous and can harm organisms since they are very aggressive. For instance they denature proteins and are hygroscopic, deadly for any living cell. So what will happen when we put these two very, very dangerous substances together?

Fig. 4 Exploding vehicle. Source
Okay, I’m exaggerating! It won't explode. Let me explain what is happening during the process called neutralisation.
Dangerous + Dangerous = Water + Salt
Let’s take our HCL and NaOH for instance. Our acid HCl will break into H+ and Cl+ and our base into Na+ and Cl-. The product will be H20 and NaCl. We all know NaCl and use it daily, it’s salt! Did our neighbor everything right? No! This would make things only worse. In a case of an emergency the best thing to do is call the doc. This story is obviously fake. Fake news. My colleague wanted to trick me.
In order to have a perfect neutralisation where the water is neutral and has a pH value of 7, you would need to add the exact same concentration of base to the acid. Secondly the reaction is strongly exothermic, means a lot of energy is released.

Fig. 5 Neutralisation. Source
Thanks for reading and sharing your time! I would love to have some feedbacks and suggestions for the next article. I will raffle 10 Steem among all who can tell me what acid you need in order to dissolve gold.
Keep steemin’,
@timsaid

