
I remember the days in school where my chemistry teacher warned us about the danger of drinking distilled water. We will suffer a painful end and nothing would save us. Ever since then I was afraid of distilled water. One day I met a friend who told me he drinks his tea only with distilled water. I couldn’t believe it. Was he making fun or was I hoaxed all these years? It sounds logical that your cell will literally explode by the distilled water. On the other hand we are made of around 65 percent water. I wanted to get to the bottom of things and started my research. Is this fact or fiction? We will find out!
The molecule of life
Water is the only chemical compound which can be naturally found in the three basic aggregate states: solid (ice), liquid (water) and gas (water vapor). It is made of hydrogen and oxygen in a ratio of 2:1. About 71% of the earths surface is covered by water. The molecule itself deserves much more than just a simple post and I might write one soon about this very interesting molecule. In this article however, we will focus on the biological processes that are determining how water is regulated in our cells. To be more accurate, we will have a look at the osmosis. The water we usually drink, whether it is from the faucet or the bottle from the supermarket has many ions. To give some examples: calcium Ca²+, Magnesium Mg²+, hydrocarbonate HCO3-. If you want to know what exactly is in your water have a look at the back of the bottle, there you will find detailed concentrations of the ions. Distilled water is free of any minerals and has no ions, it only consists of pure H20. Extracting distilled water is very easy: you boil water and let collect the condensed water separately. The condensed water is free of ions and is distilled.
To explain osmosis we will first have a look at the following illustration:
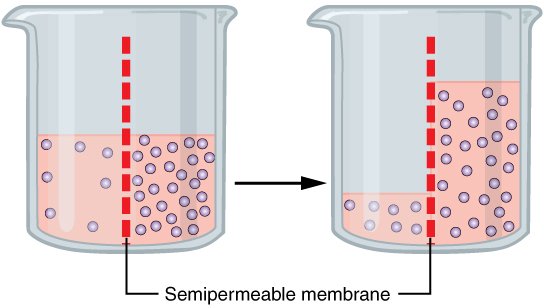
Fig.1 Schematic illustration of osmosis. Credits
We see a container with a liquid and a semipermeable membrane inside. Let us assume the liquid is red colored water and the little balls represent molecules or ions. On the left side we see that the concentration of ions is not evenly distributed. The molecules however can’t move through the semipermeable membrane. Semipermeable means it is only “open” to certain molecules. Applied to our cells this can mean for instance that no ions can move through this natural barrier. Water is able to move through the semipermeable membrane. In order to balance the concentration of each side of the membrane water is moving towards the part where the concentration of ions is higher. Now on the right side we can see an evenly distributed concentration. Concentration can be defined as the quotient of the amount of substance divided by the volume. With this definition you will now see that the concentrations within the left container is higher on the right side of the membrane. In the left container however the concentration is even.
Now replace the container with our blood cells.

Fig.2 Blood cells in different mediums. Credits
Our blood cells move in isotonic blood plasma. Isotonic means that the osmotic pressure is even. The concentration in and outside the cell is the same. Then there is hypo and hypertonic. Hypertonic means that the concentrations of ions outside our cell is higher and water flows out of the cell. On the other side hypotonic means the concentration of ions inside the cell is higher and water flows in. What would happen if we ONLY drink distilled water? Our blood plasma for instance would have a lower concentrations of ions and water moves into our blood cells, making them explode. A deadly effect.
The dose makes the poison
Like many things in life the dose makes the poison. Salt for instance is essential, in too high concentrations however deadly. (The first who gives the correct answer on what salty water does gets 10 SP from me). Same goes to distilled water. A cup of distilled water or two are fine, if however you would drink too much if might have consequences. Again, even too much drinking water is dangerous. A balanced nutrition is the key. The distilled water mixes with the minerals from our food in our stomach and thus would have no impact on our body.
No badge this time. Distilled water can cause death but it is very unlikely to happen, means this myth is partly true.
I hope you enjoyed this episode of Myth or Fact. The next one will follow tomorrow.
-Tim
