
Most of us might have seen the opening sequence of the Simpsons where we can see Homer working with a green glowing rod. It is known that he works in a nuclear power plant and the green glowing material is supposed to be a fuel rod. Not only in the Simpsons but in many books, movies or games the typical green glow occurs wherever radioactivity plays a role. 9 from 10 of my friends I asked said anything radioactive glows green.
Is it true that radiation and nuclear material glow green or is this just another myth? Today we will find out. We will have an insight at what radioactivity is and answer few questions.
Radioactive decay
Radioactivity can be described as the processes of unstable atomic nuclei decaying into other lighter nuclei and energy carried away by photons. The emission of particles cause the radionuclide to change into another nucleus, until a stable atom is formed. There are different kinds of radioactive decay and we will focus on the three basic decays here: alpha-decay, beta-decay and gamma radiation.
As described radioactivity is the decay of unstable nuclei. Atoms are made of electrons, protons and neutrons. The electrons have a negative charge, protons a positive and neutrons have no charge. Protons and neutrons together build the nuclei while the electron forms the atomic shell. We know that unlike charges attract each other while same charges repel. This is also known as the Coulomb law. In order to keep the nucleus together there is another force which is stronger than the Coulomb force. The nuclear force keeps the nucleus together. The stability of a nuclei is determined by the ratio of protons to neutrons as well as the total number of nucleons. Light nuclei are stable with a ratio of 1:1. The heavy stable nuclei have a ratio of 1,5:1. Heavy nuclei need more neutrons in order to reduce the repel between the protons.
Nucleus with a different amount of neutrons but same amount of protons are called isotopes. Carbon for instance has a well known isotope, C14. In nature however C14 is unstable. A good overview gives the belt of stability. N is the number of neutrons and Z the number of protons.
The colored area shows unstable isotopes that decay whereas the black shows stable isotopes. Furthermore the figure shows that nuclei with more than 20 protons need additional neutrons to stay stable. Another point we can see on the illustration is that heaviest nuclei is has 82 protons and 126 neutrons. This nuclei is Plumb-208.
Additionally nuclei are stable when they have 2, 8, 20, 28, 50, 82 protons or 2, 8, 20, 28, 50, 82, 126 neutrons. The numbers of these nucleons are called magical numbers. Any isotope with these amount are stable. There are higher magical numbers but they are not yet confirmed. If you want to know more about magical numbers I recommend you this article from @lemouth

Fig.1 Belt of stability. Showing the different kind of decay of isotopes. Credits
Now that we know why an atom can unstable, lets investigate the different kinds of decay.
Alpha, beta and gamma decay
The alpha decay is the radiation of helium nuclei, made up of 2 protons and 2 neutrons. Because this particle has no atomic shell it has a positive charge. As we can see in the figure above alpha decay occurs at heavy, unstable nuclei. After the alpha left, the mother nucleus has 4 neutrons and two protons less.
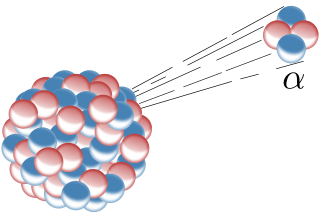
Fig.2 Schematic illustration of the alpha decay. Credits
The beta decay occurs on heavy as well as on light unstable nuclei. Because of the neutron excess, a neutron decays into a proton and electron. The proton stays in the nucleus whereas the electron is emitted. Because the proton has a higher mass it only can decay into a proton and an electron. This ionizing radiation has a negative charge since electrons are emitted. (To be accurate the neutron decays into a proton, electron and electron-antineutrino). The above described decay is also called beta- radiation. There is also a beta+ radiation where positions are emitted (imagine an electron with a positive charge)
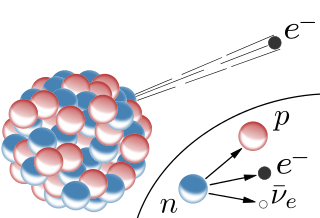
Fig.3 Schematic illustration of the beta decay. Credits
The gamma decay itself has no particle emission. In most cases a nucleus is left in an excited state after alpha or beta decay. When this nucleus gets back to a lower state it radiates penetrating, electromagnetic gamma rays. So mostly gamma radiation is observed together with alpha and beta decay.
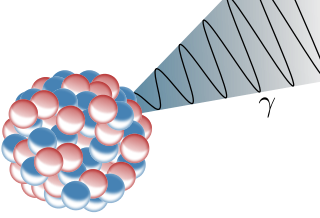
Fig.4 Schematic illustration of the gamma radiation. Credits
There are ways to shield against the different types of ionizing radiations. Alpha, beta and gamma are named in the order of their ability to penetrate material. Alpha has the lowest range and gamma the highest. To protect against alpha radiation a simple sheet of paper helps. Thin foil of aluminum can be effective against beta radiation and to stop gamma rays plumb is used. Gamma rays are dangerous because they have a high range, however alpha and beta radiation are not to be underestimated since they can have lethal effects on organisms.
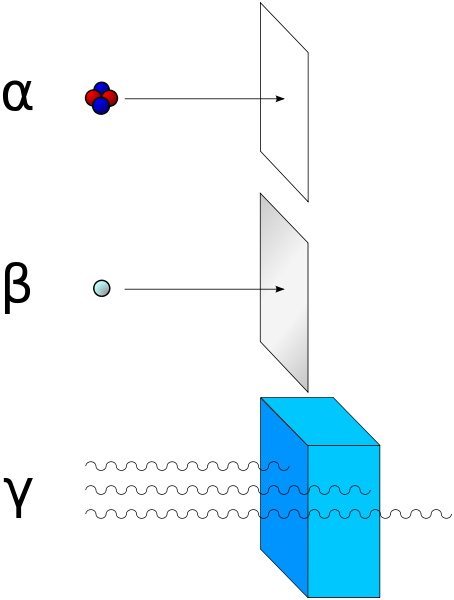
Fig.5 Alpha radiation can be blocked with paper, beta with thin aluminum foil and gamma radiation with plumb. Credits
Where does the green glow come from?
We saw that radioactive decay emits particles or gamma rays. None of these are visible to the human eye. In one my my articles about the sense of sight I describes that the human eye is only able to see electromagnetic waves with a wavelength between 380 nm and 780 nm (nm = nanometer). Gamma rays have a much smaller wavelength and are not visible.

Fig. 6. The electromagnetic spectrum. Credits
So why and where does the myth come? While radioactive material itself does not glow, it can make fluorescence or phosphorescence materials glow. Back in the 90s it was common to use radium and mix it with a phosphor that contained zinc sulfide and copper. The ionizing radiation of radium made the phosphor glow green. The advantage was a bright green light that lasts for hundreds of years and was even visible at day. The danger of ionizing radiation was not known to this time and many of the workers suffered from serious diseases like cancer. The workers are also known as “Radium Girls”.

Fig. 7. Radium dial. Credits
The idea that radioactive material glows green is just a myth. In face we can’t even see the radiation because our eyes don’t have the right receptors. There is however one kind of radiation we can see. The next picture shows the internal of a nuclear reactor. The water in the pool appears to glow blue. As we know light travels the speed of c. This is only the case in a vacuum and in water the light travels slower. The particles from the radioactive decay move faster in the water than the light and therefore do emit the so called “Cherenkov radiation”.
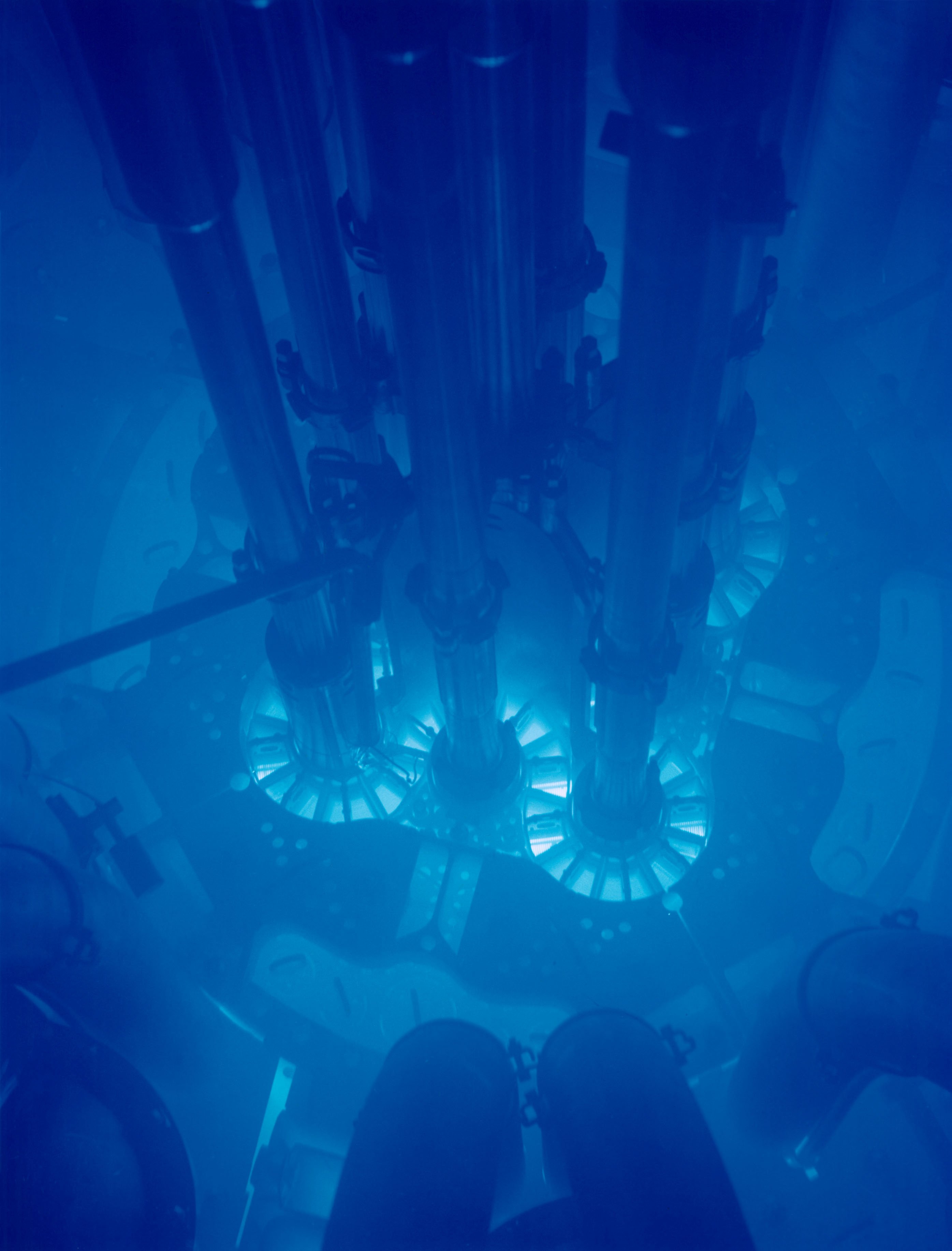
Fig. 8. Nuclear reactor from inside. Credits
This was again nothing more than just a myth. When you see something glowing green next time, don’t be afraid.

I hope you enjoyed this episode of Myth or Fact. The next one will follow tomorrow.
-Tim
