
Imagine a very cold winter day. You sit outside and watch the snow falling. Your friend asks you for a hot tea and you bring him a cup. You however decide to drink a cold, refreshing water. Suddenly your favorite movie starts and you rush into your living room and forget the drinks outside. After an hour you go back to your garden just to see that one of the drinks is frozen. To your confusion the tea was frozen whereas the water wasn’t.
Can the described situation really happen? Doesn’t it sound more consistent that the already cold water freezes first? In this episode of Myth or Fact we will investigate this question.
State of matter
As we know water does consist of H20 molecules. Water is the only natural chemical compound that occurs naturally in all three different states on earth: solid, liquid and gas. There are more states of matter, but the most well known states are these three. We will now have a look at the differences between the states on the example of water.
H20 is made of hydrogen and oxygen in a ratio of 2:1. A single drop of water contains billions of atoms! The forces between the molecules and the environmental conditions like temperature and pressure determines the state of matter.
The following picture gives a great sense about what is happening with the molecules in each state. Intermolecular forces keep the molecules together. To overcome these intermolecular forces thermal energy is required which puts the molecules in motion. Two of the main intermolecular forces are the Van der Waals forces and Hydrogen bonds.

Fig.1 Illustration of the three basic states of matter. Credits
Van der Waals forces are intermolecular forces that rely on the electrons. Electrons move around a certain orbit of an atom, however their exact location can’t be determined. Let’s get theoretical: imagine a round ball and another small ball moves around the big one. You know there is a ball orbiting but you can’t tell where. The atom it self has no charge, it’s neutral. Our electron however is negative. Dipoles are molecules that don’t share their binding electrons equal. One atom attracts the electrons stronger than the other atom. This causes the molecule to have one part with a higher density of electrons. The more dense part has a partial negative charge because of the electrons. The ability of an atom to attract electrons is called electronegativity. The stronger the electronegativity the stronger it attracts the electrons in a chemical bond.
If we have two identical atoms we have an identical electronegativity. This means the atoms share their electrons even and there is no dipole. The orbiting of the electrons causes a difference in the local density of the electrons. This difference can induce a transient dipole. This transient dipole then can induce other dipoles in neighbor molecules. Due to this behavior a weak intermolecular force acts which keeps the molecules together.
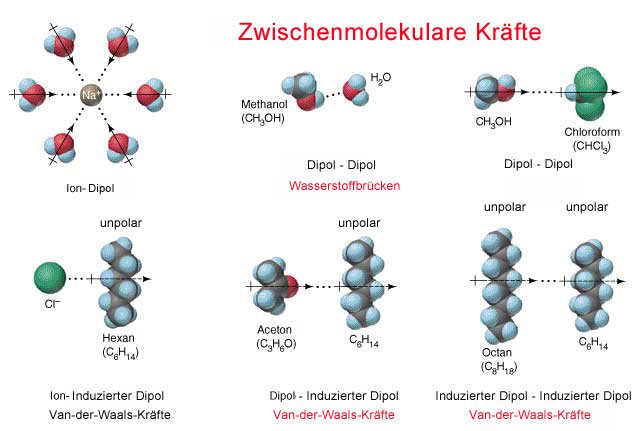
Fig.2 Different intermolecular forces. Credits 1 2
The stronger the forces the more “solid” the substance. In solids there are covalent and ionic forces. These are the strongest molecular forces that keep molecules tight together and doesn’t allow them to move. In a liquid we have less stronger forces like the Van da Waals forces and Hydrogen bonds which allow the molecules to move. In a gas however, the forces are very weak which allows the molecules to move even more and expand. The volume the substance takes is higher the weaker the forces are. Gases take more volume than liquids do.
In case of water we have the following situation. Water in solid matter is known as ice. In this state we have many hydrogen bonds and an arranged structure of the molecules. Hydrogen bonds are forces between two polar molecules. To be more accurate hydrogen bonds are intermolecular forces between hydrogen atoms and free electron pairs. Each H20 molecule can make up to four hydrogen bonds with other H20 molecules.
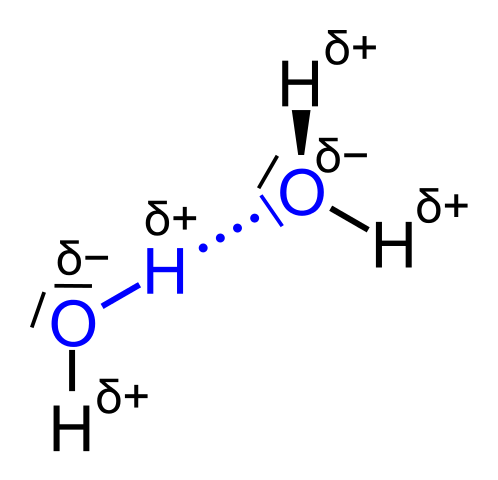
Fig.3 Hydrogen bond between two H2O molecules . Credits

Fig.4 Hydrogen bonds. Credits
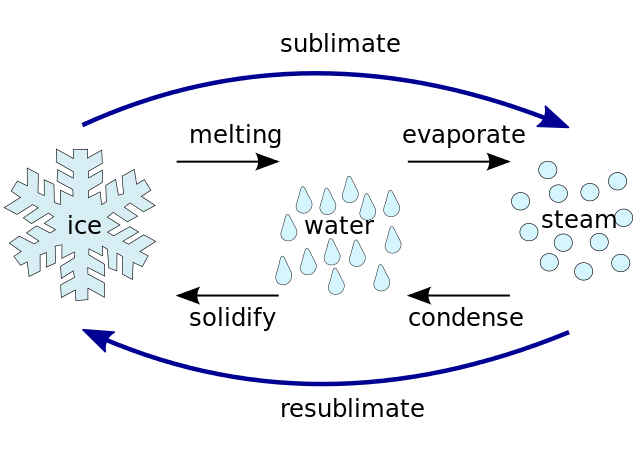
Fig.5 Different aggregate phase and terms. Credits
Hot or cold? What freezes faster?
The answer is that hot water freezes faster. The phenomenon is called after it’s founder Erasto Mpemba. There are several ideas why this is the case. The process of water turning into steam is called evaporation. The warmer the water the faster this process. Because the water in the cup loses more volume than the cold due to the evaporation, effectively we have less water that can freeze. If you put a thin layer of oil on top of the water surface the water freezes much slower. Another reason is convection. Hot water has a lower density than cold water. The areas that cool first are the top and the surface between the glass and the liquid. In these areas the water is colder than in the middle of the glass. This difference in density causes the water inside to move up. The movement therefore causes additional thermal exchange and accelerate the process of freezing.

Yes, it’s true. Hot water freezes faster than cold does!
I hope you enjoyed this episode of Myth or Fact and thanks for reading. The next one will follow tomorrow.
-Tim
