
Many things around us and with us happen we take for granted. We see, feel, taste, hear and smell unknowingly how it works. As we were children we called anything into question and now accept reality as it is. We lost the magic in all these wonderful things. This series addresses the wonder of natures and will bring its magic back to light. The first part of the Life Explorers - The Human Senses starts with the sense of sight. We see forms and colors, see bright and dark. But why?
To be able to answer this question we first need to have look at the nature off light. Light is a form of electromagnetic (EM) radiation, to be accurate we need to speak of visible light because only a small portion of the actual EM spectrum is visible to the human eye. The following illustration shows the EM spectrum. Frequency and wavelength are inversely proportional, the shorter the wavelength the higher the frequency. With higher frequencies the amount of energy is rising, this is for instance the reason why Gamma Rays are deadly. The high energy of the gamma rays are devastating for biological cells as they are ionizing and cause secondary radiation like X-Rays or electron diffraction. E= h • f is formula which depicts the proportionality of E energy and f frequency (h stands for the Planck constant). Here a little example for the energy of a short and a long wavelength.
| red | E=h • f = 6,626 070 040 · 10−34 J*s * 384 THz | 1,59 eV |
| violet | E=h • f = 6,626 070 040 · 10−34 J*s * 789 THz | 3,26 eV |
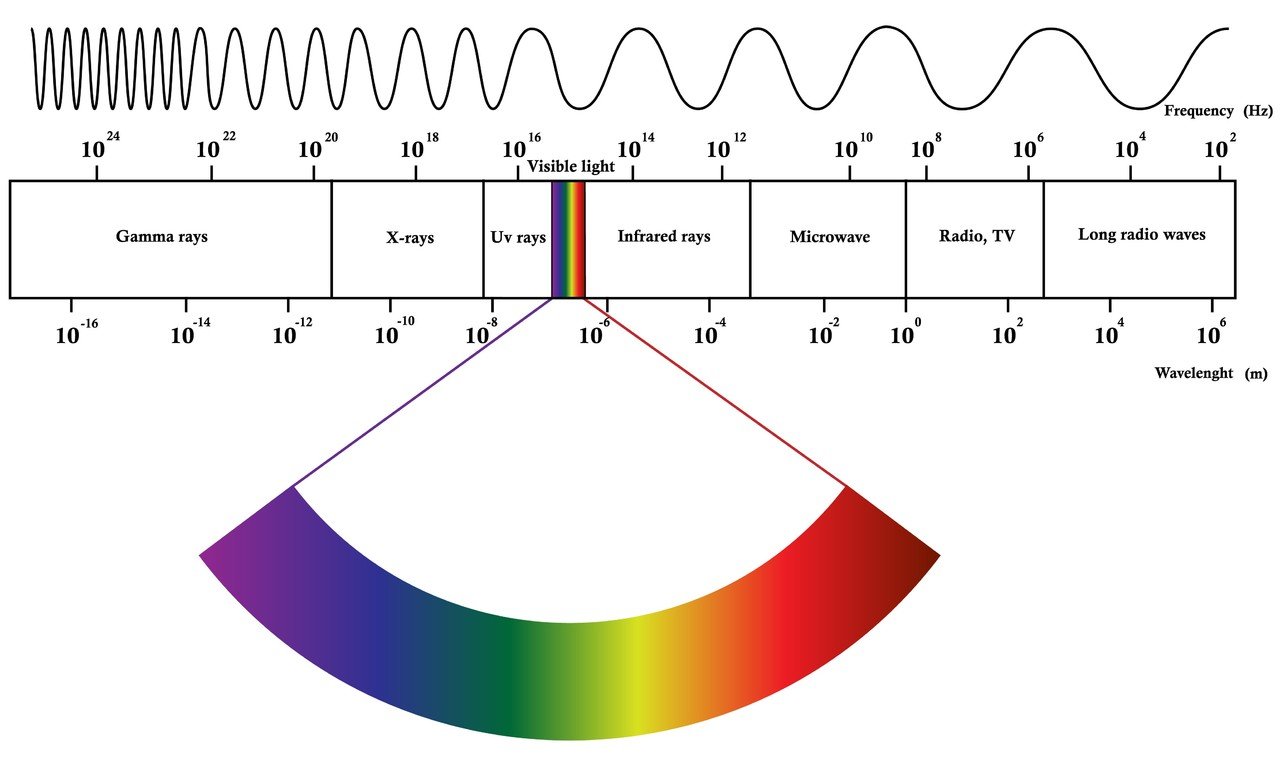
As shown above the visible light is only a tiny portion of the EM spectrum. The human eye is able to see colors from red to violet, which is equivalent to wavelengths from 780nm to 380nm (nm = nanometer).
Why do is the Steemit logo blue and not red?

Sure because @dantheman and @ned decided so :) But on a scientific base, how come we see blue and not another color?
Now that we know what visible light is let’s have a look at the interaction of EM radiation and the objects we see. The actual color we perceive depends on the physical principle of reflection and adsorption.
Most objects reflect light but before that happens they adsorb part of the light they receive. The wavelength they adsorb determines what your eye will process later. For instance when all wavelengths expect red (780nm) are adsorbed the object will reflect red. Same goes for a blue steemit logo for instance. The logo absorbs all wavelengths expect the blue.
More than one wavelength can be reflected the same time, this therefore is why we see shades of the same color.
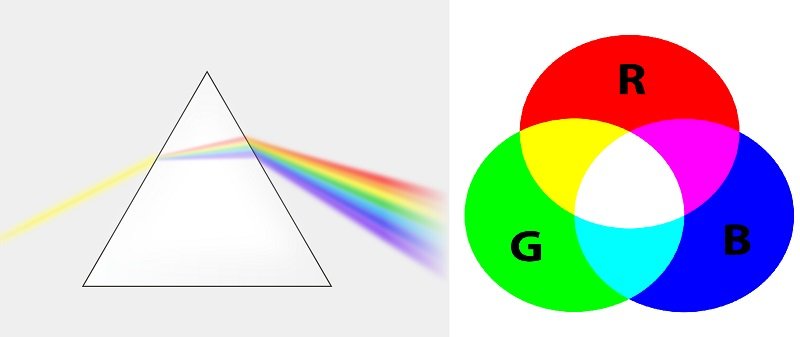
We can see purple color when blue and red are reflected wavelengths. We see white when an object absorbs no light.
Isaac Newton showed that white light contains all colors. He used a prism to separate the colors of spectrum of visible light.
How does reflection and adsorption work?
To understand how adsorption works we need to have a closer look into the chemistry of molecules. As described light hitting an object, here molecule, is adsorbed and we see the complementary color as a result. The following abbreviations will be used: HOMO = Highest occupied molecule orbital and LUMO = Lowest unoccupied molecule orbital. Carrots are orange, but why?

Electrons are moving in a certain space around the nucleus, called orbitals. The energy of light promotes an electron from a bonding or non-bonding orbital into an empty anti-bonding orbital, from HOMO to LUMO. The gap between these two orbitals is equivalent to the amount of energy that is needed to promote the electrons. A larger gap therefore requires light with more energy, for instance blue or violet. The chemical molecule that gives the carrot its orange color is called beta-carotene. Beta-carotene absorbs light between 400nm and 500nm with a peak at 470nm. Having a look at our additive color schema we will see that the complementary color of blue is yellow. Now is that not only blue, but a wide range of light between 400nm and 500nm is absorbed.
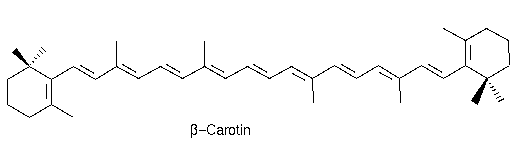
So when light hits the electrons of beta-carotene the electrons move from HOMO to LUMO and adsorb the light between 400nm and 500nm. This is the wavelength needed to promote the electron with the exact amount to energy needed.
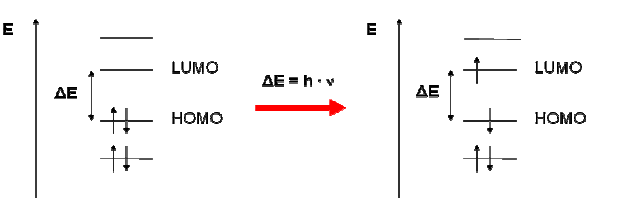
Note: The electron moves back from LUMO to HOMO. The energy from the light used to promote the electron from HOMO to LUMO can be emitted in form of radiation or heat. In summer we all notice that black material gets warmer than bright. This is because the complete wavelength of the VIS is absorbed and emitted as heat.
Transforming waves to information
Now that we know how the light is absorbed by objects we need to examine how our eyes work. Without the human eye we wouldn’t see anything, that’s for sure. But how are we able to distinguish colors and brightness? Our retina has approximately 6 million cones and 120 million rods. When the reflected light hits our retina these cells become active.
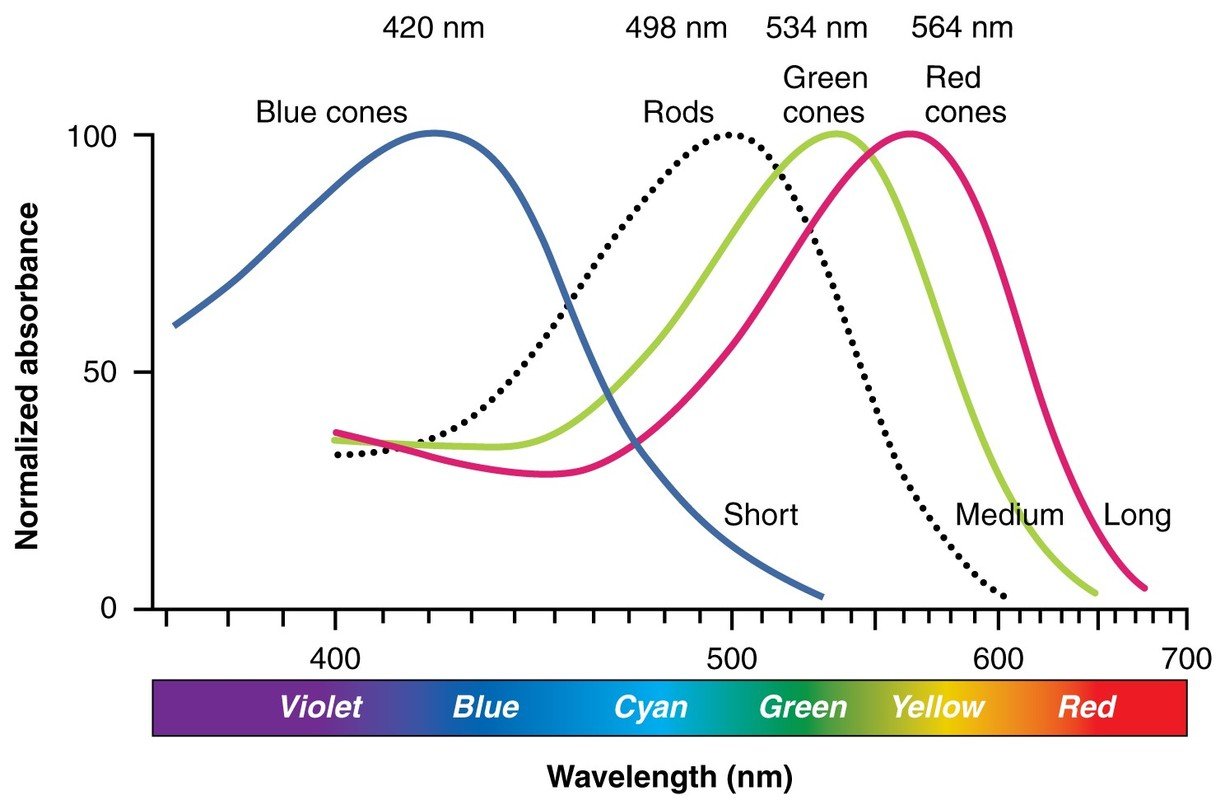
We have three different type of cone cells, S(-hort), M-(medium) and L-(ong) cones. Short, medium and long refer to the wavelength the cell is responding to. The rods however can work with different wavelengths and thus do not matter when it comes to color. They are responsible for brightness and become active when there is less light. While the cones are active and working we speak of photopic vision, responsible for color sight. Scotopic vision is the terminus for sight in low light and only seeing in shades of gray.
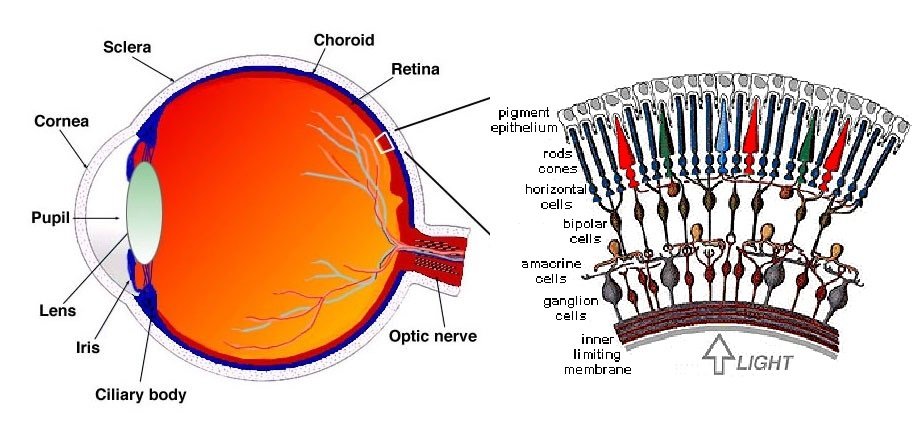
As described the human retina has three kinds of cones. Each sensitive to a different wavelength. The following illustrations depicts the relation of wavelength and cones. These three cones together make it possible that we see colors. By combining cones we are able to see over 200 million different colors.
So when light hits a cone it becomes activated. How does this activation work?
This is a very complex biochemical process I will try to explain without going too much into detail since we could do several posts just about this process. However we will have a look at the basic.
Both, cones and rods contain a light-sensitive receptor protein called Rhodopsin, also known as visual purple. Under the influence of light Rhodopsin decomposes into Opsin and trans-Retinal. This process repeats and creates a cascade. The end of the processes results in regulating a molecule named Glutamat. The cells behind the retina turn these signals into nerve impulses to make it processable for the human brain.
Thank you for reading the first part of Life Explorers - The Human Senses, Don't miss the next part.
Do you have an questions or suggestions? Want to know about a scientific topic? Leave a comment.
Tim Said
