
Soap is one of those daily essentials that we depend on, but never really think about.
It's being used in so many different ways - laundry detergent, dishwashing liquid, shower gel, specific soap for our face, hand soap, shampoo...
But have you ever asked yourself how soap actually works?
How Soap works
Soap is essentially made up of a combination of fatty acids and base ingredients, which create a so-called saponification reaction when they're together.
When you use soap, 2 things happen:
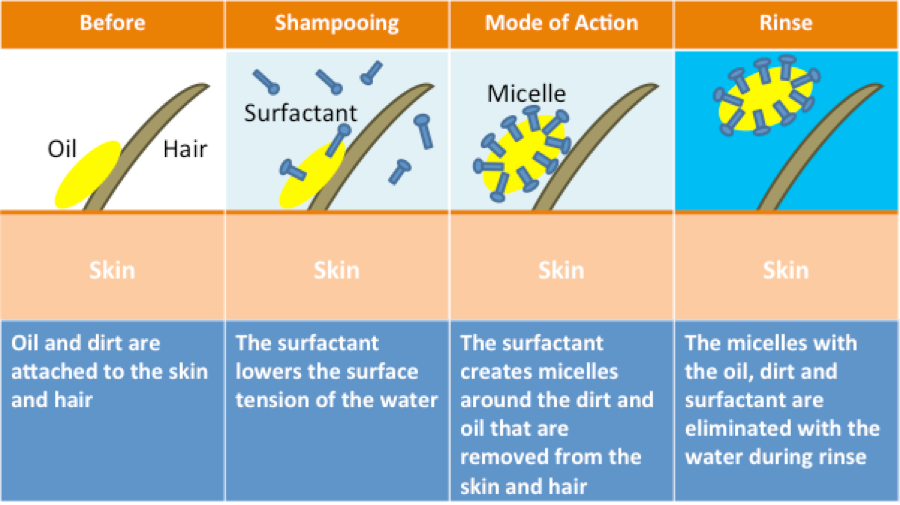
1) The soap reduces the surface tension of the water.
The surface tension is what makes water form drops. This behaviour slows down the process, so by reducing the tension you are basically making the water "wetter" and speeding up the cleaning process.
2) The molecules wash away dirt.
The particles in the soap have two ends. One of them is hydrophilic (water-loving), while the other end is hydrophobic (water-fearing, but oil-loving). And this is essentially the secret of soap!
When you wash your hands, the hydrophobic ends adhere to the oil, dirt and germs.
If you then rinse it off with water, the soap gets pulled away due to the hydrophilic parts that stick to the water - making it possible for you to wash away the soap and the dirt and oils that stick to it.
Of course, the mechanical process of rubbing your hands helps getting the dirt and oil off of your skin even more.
So apart from popular belief, soap doesn't actually kill bacteria, it just washes it away.
And since water and oil don't mix, water alone couldn't do that as quickly, or as easily.
Scientists suggest that you should wash your hands for at least 20 seconds, not neglecting the space in between your fingers and along the fingernails, and then rinsing while continuously rubbing your hands.

Kinds of Soap
Depending on what kind of soap it should be, a lot of other ingredients can be added to this basic recipe.
This could be abrasives to remove more stubborn dirt, water softeners, skin moisturizers, fragrances, enzymes that help with biological stains like blood, ingredients that adjust the pH levels or ingredients that alter the texture of the soap, for example making it more foamy or more gel-like.
Shampoo is of course also a form of soap, and it works with the same basic principle where one end of the molecule sticks to the dirt and oil, while the other end sticks to the water, and everything gets washed away.
Shampoo is a more complex version of soap because for one it can't dry out the scalp but still needs to be cleansing, and also it can have many more properties like removing dandruff, treating colored hair etc.
Antibacterial Soap
Antibacterial soap is its own, special kind of soap that fights the bacteria instead of just washing it away.
This might seem more effective, but many studies have already proven that it's not neccessary for "normal" people to use antibacterial soap. It's more aggressive to the skin, and also increases the resistance of bacteria overall.
Apart from that, you would have to wash your hands for about 2 minutes until the antibacterial components would have killed all the bacteria on your hands.
The History of Soap
Soap has actually been invented thousands of years ago.
Our ancestors in Babylon and ancient Egypt were already using a mixture of animal and vegetable oils in combination with alkaline salts.
A Roman legend says that they discovered soap because of Mount Sapo:
This was a holy mountain where the romans would sacrifice animals as a gift to the gods.
When it rained, the fats and oils from the sacrificed were washed down the mountain in combination with the alkaline ashes from the fires.
This mixture was washed down the mountain and into the the river Tiber, and the people found out that this was helping them clean their clothes.

Images: 1, 2, 3, 4, 5, Sources: 1, 2, 3
- Instagram -

Sirwinchester

