In the scientific world several discoveries happened by accident for example in 1945 Percy Spencer was messing around with the energy sources of his radar equipment when he noticed that a chocolate bar he had in his pants was melting (not very smart of him to keep a chocolate bar in his pants). Anyway, this accident lead to the invention of the microwave.
There were even more fortuitous episodes, one accident won the Nobel Prize for Elie Metchnikoff. He was observing a starfish under a microscope (you can tell they did not have television at the time), but at lunch time he left the starfish unattended. This was a great opportunity for his kids to mess around with it and poke thorns into it (kids can be mean). When Metchnikoff came back it looked like the show was pretty much over but luckily, he decided to look at the starfish for a last time and he noticed that the cells of the starfish were concentrated around the thorns. Later, he found out that these cells were leukocytes and that gathered around the foreign body (the thorns) to devour the foreign particles. Metchnikoff described this phenomenon as phagocytosis, a major defense mechanism of our body. For this, he was considered the father of immunology and in 1908 he received the Nobel Prize.

Today I want to talk about another fortuitous discovery made by Jan van Deursen, even if he wasn’t poking a starfish with thorns. Van Deursen and his colleagues at the Mayo clinic in Minnesota, were creating transgenic mice, engineered to develop tumors more easily so to test different cures for cancer research. But, instead of developing cancer, his mice were just looking awful, they had thin fur and cataract in their eyes, they were pretty much ageing awfully fast. After some experiments they realized that this was due to the presence of strange cells that would not divide but that would not die, we could say they created mice with zombie cells (Baker et al., 2008). In science we use a less picturesque name for these type of cells, we simply call them senescent cells.
What are senescent cells and why do they form?
First of all let’s say that the concept of senescent cells is not new, Leonard Hayflick coined this term in 1961 where he suggested that there could be ageing at the cellular level (Hayflick & Moorhead, 1961). At the time, little research was conducted on ageing and he was ridiculed and called an idiot by his colleagues for making this observation. Today we know he was onto something, during their life, cells accumulate damage. If the damage is at the DNA level, a cell could pass along the damage to its progeny and this could be a disaster for our body. For this reason, cells have developed defense mechanisms to prevent damaged cells to create other cells bearing the same damage. The options are two: a cell either dies or becomes senescent, which means it stops dividing (Kirkland & Tchkonia, 2017). For years we thought senescent cells were not very important, since they don’t divide why bother studying them (Scudellari, 2017)?
Today we know there is much more into it, there is also a “dark side” to senescence. In 2008, a bunch of studies revealed that senescent cells don’t just stay quiet as we previously thought, but actually produce pro-inflammatory cytokines, growth factors and proteases which combined, cause inflammation in the nearby tissues (Acosta et al., 2008; Coppé et al., 2008; Kuilman et al., 2008). This activity is also known as “senescence associated secretory phenotype (SASP) (Coppé et al., 2008).
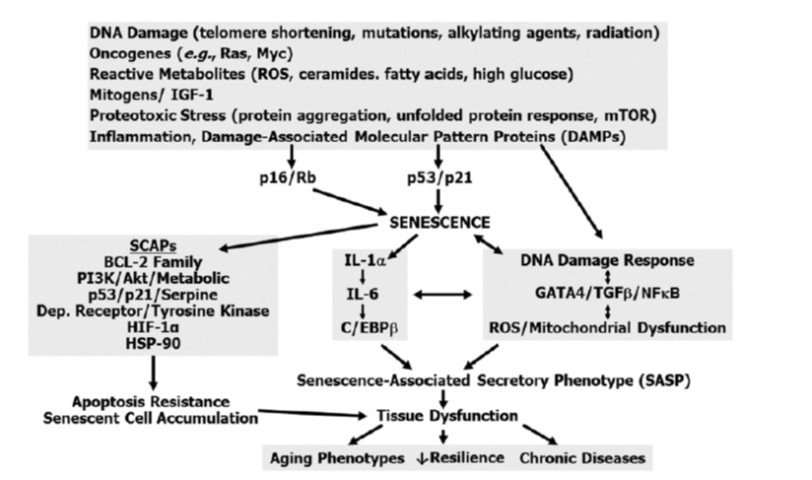
Schematic depicting pathways associated with senescence - adapted from (Kirkland & Tchkonia, 2017)
When a tissue is young and healthy, actually a few senescent cells secreting SASP could even stimulate tissue repair and instruct the immune system to get rid of the zombie cells (Scudellari, 2017). However, with ageing and the accumulation of a number of senescent cells we can start observing the insurgence of chronic inflammation that could lead to pathologies such as osteoarthritis (inflammation of the joints) or atherosclerosis (hardening of the arteries). Moreover, these zombie cells show a tissue dependent behavior, depending on their tissue of origin they will secrete different SASP and trigger different responses, this makes it difficult to counteract their action with a universal cure.

OK, so zombie cells cause chronic inflammation, is that all?
Not exactly, we did not finish our story yet. So van Deursen found that the presence of these zombie cells was accelerating ageing in mice (Baker et al., 2008). In 2011 the same team tried to get rid of these zombie cells to see what would happen, they found that these zombie cells accumulate in ageing organs and by eliminating them it is possible to alleviate and even prevent the insurgency of some illnesses (Baker et al., 2011). After these surprising findings, more studies followed and they all showed similar results, by eliminating zombie cells it was possible to restore mice fitness, fur thickness and kidney function(Baar et al., 2017). Also the symptoms of lung diseases could be alleviated (Schafer et al., 2017) and partially restore damaged cartilage (Jeon et al., 2017). A study conducted in 2016 even found that the lifespan of mice could be extended 25% by eliminating senescent cells (Baker et al., 2016).
Following these discoveries a new class of drugs is being developed called senolytics, which means these are molecules that aim at destroying senescent (zombie) cells. However, because the Food and Drug Administration has not labelled ageing as a condition in need of treatment, it is still challenging to obtain funding to conduct research on senescent cells.
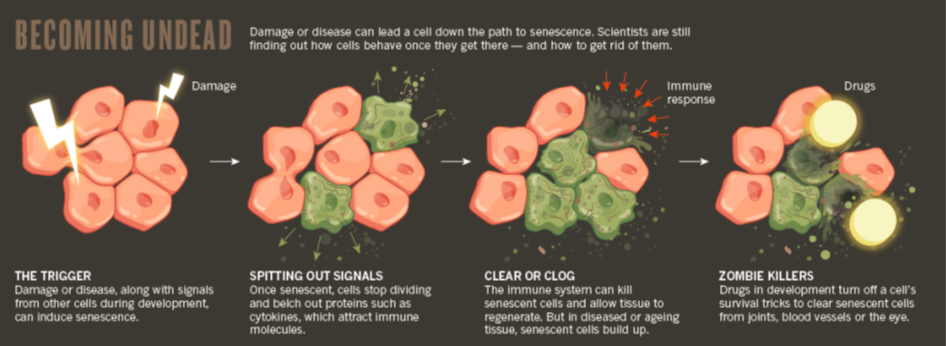
Diagram depicting how zombie cells create inflammation and how they can be dealth with - adapted from (Scudellari, 2017)
What would it take to kill senescent cells?
To recap, senescent cells are cells in their twilight, they are not dead yet, but they are not so easy to kill neither. In fact, they rely on several (probably around six) signaling pathways to defy death (Fuhrmann-Stroissnigg et al., 2017; Zhu et al., 2015). Theoretically, a drug to be considered senolytic should disrupt these pathways. So far, 14 different molecules have been described in literature to possess such properties (Chang et al., 2016; Schafer et al., 2017; Yosef et al., 2016; Zhu et al., 2016). Since senescent cells behave differently, probably we won’t find a single drug capable of being effective on all of them, but combination of different senolytics may be a viable option.
There are some advantages in a hypothetical senolytics therapy, for instance we don’t need to eliminate all zombie cells, just killing a few will already show clinical benefits (Scudellari, 2017). Moreover, the therapy would not interfere with the formation of senescent cells, which means that our cells will maintain this tumor suppressing feature and become senescent if they undergo DNA damage. This is crucial as a senolytic therapy will likely have small side effects, also considering that it would be a “hit and run” type of therapy. This means that the patient won’t be under senolytic administration for prolonged periods of time, but just once or twice a year.
Just to be clear, senolytic therapy won’t stop ageing but it may alleviate some of its symptoms. If we really want to stop ageing first we need to understand what ageing really is.
References:
- Acosta, J. C., O’Loghlen, A., Banito, A., Guijarro, M. V, Augert, A., Raguz, S., … Gil, J. (2008). Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell, 133(6), 1006–18. https://doi.org/10.1016/j.cell.2008.03.038
- Baar, M. P., Brandt, R. M. C., Putavet, D. A., Klein, J. D. D., Derks, K. W. J., Bourgeois, B. R. M., … de Keizer, P. L. J. (2017). Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell, 169(1), 132–147.e16. https://doi.org/10.1016/j.cell.2017.02.031
- Baker, D. J., Childs, B. G., Durik, M., Wijers, M. E., Sieben, C. J., Zhong, J., … van Deursen, J. M. (2016). Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature, 530(7589), 184–9. https://doi.org/10.1038/nature16932
- Baker, D. J., Perez-Terzic, C., Jin, F., Pitel, K. S., Pitel, K., Niederländer, N. J., … van Deursen, J. M. (2008). Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nature Cell Biology, 10(7), 825–36. https://doi.org/10.1038/ncb1744
- Baker, D. J., Wijshake, T., Tchkonia, T., LeBrasseur, N. K., Childs, B. G., van de Sluis, B., … van Deursen, J. M. (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature, 479(7372), 232–6. https://doi.org/10.1038/nature10600
- Chang, J., Wang, Y., Shao, L., Laberge, R.-M., Demaria, M., Campisi, J., … Zhou, D. (2016). Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine, 22(1), 78–83. https://doi.org/10.1038/nm.4010
- Coppé, J.-P., Patil, C. K., Rodier, F., Sun, Y., Muñoz, D. P., Goldstein, J., … Campisi, J. (2008). Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biology, 6(12), e301. https://doi.org/10.1371/journal.pbio.0060301
- Fuhrmann-Stroissnigg, H., Ling, Y. Y., Zhao, J., McGowan, S. J., Zhu, Y., Brooks, R. W., … Robbins, P. D. (2017). Identification of HSP90 inhibitors as a novel class of senolytics. Nature Communications, 8(1), 422. https://doi.org/10.1038/s41467-017-00314-z
- Hayflick, L., & Moorhead, P. S. (1961). The serial cultivation of human diploid cell strains. Experimental Cell Research, 25(3), 585–621. https://doi.org/10.1016/0014-4827(61)90192-6
- Jeon, O. H., Kim, C., Laberge, R.-M., Demaria, M., Rathod, S., Vasserot, A. P., … Elisseeff, J. H. (2017). Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nature Medicine, 23(6), 775–781. https://doi.org/10.1038/nm.4324
- Kirkland, J. L., & Tchkonia, T. (2017). Cellular Senescence: A Translational Perspective. EBioMedicine, 21, 21–28. https://doi.org/10.1016/j.ebiom.2017.04.013
- Kuilman, T., Michaloglou, C., Vredeveld, L. C. W., Douma, S., van Doorn, R., Desmet, C. J., … Peeper, D. S. (2008). Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell, 133(6), 1019–31. https://doi.org/10.1016/j.cell.2008.03.039
- Schafer, M. J., White, T. A., Iijima, K., Haak, A. J., Ligresti, G., Atkinson, E. J., … LeBrasseur, N. K. (2017). Cellular senescence mediates fibrotic pulmonary disease. Nature Communications, 8, 14532. https://doi.org/10.1038/ncomms14532
- Scudellari, M. (2017). To stay young, kill zombie cells. Nature, 550(7677), 448–450. https://doi.org/10.1038/550448a
- Yosef, R., Pilpel, N., Tokarsky-Amiel, R., Biran, A., Ovadya, Y., Cohen, S., … Krizhanovsky, V. (2016). Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nature Communications, 7, 11190. https://doi.org/10.1038/ncomms11190
- Zhu, Y., Tchkonia, T., Fuhrmann-Stroissnigg, H., Dai, H. M., Ling, Y. Y., Stout, M. B., … Kirkland, J. L. (2016). Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell, 15(3), 428–35. https://doi.org/10.1111/acel.12445
- Zhu, Y., Tchkonia, T., Pirtskhalava, T., Gower, A. C., Ding, H., Giorgadze, N., … Kirkland, J. L. (2015). The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell, 14(4), 644–58. https://doi.org/10.1111/acel.12344
