These posts are not for foraging. They are intended for entertainment and intellectual satisfaction only. These posts are not a field guide nor comprehensive in any way - their accuracy is not assured in any way. Do not eat wild mushrooms unless you are a professional, have substantial professional assistance or have a wealth of personal experience with a specific species. Do not make any foraging decisions based on these posts. To do so could be dangerous or life threatening.
These Posts Contains No Information Regarding Edibility Or Toxicity

What is a chemical reagent?
Google provides:
A substance or mixture for use in chemical analysis or other reactions.
That's a pretty broad definition. Wikipedia does a slightly more explanatory job:
...[A] substance or compound added to a system to cause a chemical reaction, or added to test if a reaction occurs.
Finally, lets take a look at good ole Merriam-Webster:
[A] substance used (as in detecting or measuring a component, in preparing a product, or in developing photographs) because of its chemical or biological activity.
From a layman's perspective - which is to say, my own perspective - I think its best to take an amalgam of all the above definitions. Or just ignore them all and take a real life example.

Testing a silver bar
Let's say you just bought a .999 pure silver bar. But you don't really trust the dealer you bought it from and you want to test it to make sure it really is pure silver.
So, in this example, you might make or buy a reagent called Schwerter's solution - which is potassium dichromate mixed with nitric acid and water.
If you apply a small amount of Schwerter's solution to a piece of metal, it will tell you, by way of the presence, or lack, of a specific visual chemical reaction, what kind of metal is present.
Schwerter's solution would be particularly helpful in testing for pure silver, because when you put a drop of the solution onto the silver bar, it should turn bright, blood red. If the drop of solution is a dark red, but not bright red, then you might have sterling silver, or .925 pure silver. If it is just brown, you might have even less pure silver - and if its green, you've really been ripped off and have a bar that is 50% silver, at best.
The point is, by seeing how Schwerter's solution reacts with a piece of metal, you can then glean information about the nature of the metal itself. For mycological purposes, a chemical reagent like KOH or Ammonia can play the same role, just applied to mushrooms.
Later on we will see another great example of a reagent when we use PH strips to test the PH of distilled water and our final KOH solution.
For now, just know that the reaction, or non-reaction, of KOH with certain mushrooms can provide useful information. Now that we know - generally - what a reagent does, lets make a solution of our own.

This is high quality KOH, or Potassium Hydroxide
When I say high quality, I mean it is 90% pure KOH. KOH is also known as potash and is an extremely base chemical. In case you don't remember from high school chemistry, when something is "base" it has a very high PH, as opposed to when something is "acidic" and has a very low PH. Both sides of the PH spectrum are very caustic, and can easily cause chemical burns, blindness, and other awful side effects if you mishandles them.
KOH has a bunch of industrial uses, including the harvesting of phosphate and cyanide. More benignly, KOH is also used in the creation of liquid soaps - perhaps most famously Dr.Bronner's. Although I do make my own soap, I tend to prefer a solid bar, which requires the use of Sodium Hydroxide instead of Potassium Hydroxide. But that's a story for another day.
For our purposes, KOH serves as a base reagent which can assist in the identification of specific mushroom species based on the reactivity, or non-reactivity, of their flesh, gills or stem to a KOH solution. The details of the chemical reaction which occurs for any given species isn't particularly important - what's important is viewing the visual results, or lack thereof, very carefully.
But we're getting ahead of ourselves. First we need to make an aqueous KOH solution, and for that, we need roughly PH neutral and uncontaminated distilled water.
Lets pour some out into a pyrex mixing cup

You may not need a full 100g of water, but it sure makes the math simpler
The goal here is to get a KOH solution that falls within the %3-5 KOH range by weight. Since I anticipate not using the solution all that often, I opted to head closer to the %5 range in the hope that the solution might last longer overall.
To get roughly a 5% solution by weight, I needed to take into account the fact that the KOH I have is only 90% pure. After doing a little math (which I admit took longer than I hoped and required double checking on the internet) I figured that I needed 5.5 grams of KOH per 100 grams or ml of water. *Like I said, to keep the math simple, I poured in 100 grams of water.
You'll note that in fact I poured in 101 grams of water. For our purposes, this margin of error is insignificant because the end product will still fall happily within the %3-5 range. However, if we were working in a lab on a project that required more precision, the 1 gram might be more important. But in that case, I'd have a better scale.
Before we confirm the KOH solution is base at the end of this post, first we need to confirm that the distilled water isn't unduly base or acidic

Looking good!
PH strips are saturated with a reagent of its own that provides the user with relevant information about the tested substance based on the color change of the PH strip. You can see the relevant color scale in the picture above. The strip in that picture has been dipped into the distilled water, and as you can see, the color falls pretty squarely in the 6-7 range, which is right about in the middle of the PH spectrum. This is also exactly what we want and expect from distilled water.
Now, in a small ceramic bowl, let's measure out 5 grams of KOH

KOH presents as small flakes until it is dissolved into a liquid
At this point we really ought to talk about safety. KOH is a very caustic substance, and so you want to make sure you have adequate eye and hand protection, and that you are working in a well ventilated space to avoid any fumes that may be produced when you mix the KOH into the water.
I am wearing plastic goggles when I do this work, as well as my soap making. I am also wearing gloves specifically rated excellent against KOH, NaOH and other base chemicals. As you can see in the photo at the top, those gloves are quite long and extend far up my arm.
Keep in mind, KOH is not the most dangerous substance on Earth or anything - but I tend to go overboard where safety is involved, and I suggest you do the same.
Once you're safety gear is on, slowly pour the KOH flakes into a non-reactive container, in this case a ceramic bowl, until you get to 5 grams. Now, I said I needed 5.5 grams to get a %5 solution - but my scale is not that accurate. So, I decided to opt for 5grams instead - and to avoid getting over 5.5 grams, I got the scale to four grams, and then added flakes one at a time, until I just broke 5grams.
Now for the magic moment, mixing!

It's important, when you mix KOH with water, to slowly pour the KOH into the water and NOT the other way around.
When KOH reacts with H20 an exothermic reactions occurs and the solution produces heat. If you pour the KOH slowly into the water, the reaction tends to occur in a safe, controlled way. The solution will heat up and create some fumes, but it should not spurt or sputter.
However, if you add the water to the KOH flakes, you run the risk of getting a significantly more violent reaction, and also accidentally propelling the light, now wet and reactive flakes, out of the vessel your using, which presents an added chemical danger.
So, with safety gear in place, and with good ventilation flowing, slowly pour the KOH into the distilled water. Then, with a non-reactive object, stir the solution until the flakes disappear completely and the fluid is clear. In this case I used a stainless steel spoon, but an HDPE implement would be more ideal.
Now we get to where those gloves really start to pay dividends - filling the dropper bottle

Carefully and slowly pour the KOH solution into the dropper bottle
More important information here. These bottles, and those funnels, are all made of HDPE, or High Density Polyethylene. It's very important, when working and storing caustic chemicals, to make sure you use the right storage materials. The internet is a great resource for this kind of information..
As it turns out, HDPE is a good choice for storage of caustically base chemicals like KOH. Glass, on the otherhand is less ideal and may deteriorate or weaken. Even worse would be different plastics - for instance the PET used in soda bottles - which would break down in the caustic solution fairly quickly, and cause a hazardous spill.
Thankfully, I've done my homework and have the proper HDPE storage bottles. Very carefully, take one of the HDPE funnels, place it into the opening of one of the droppers, and slowly pour the solution in to fill the bottle. You really should do this on a non reactive surface, stainless steel or HDPE would be ideal. Not aluminum foil, as that reacts with aluminum, causing it to dissolve.
In this case, I just used the wood of my cutting board. This isn't dangerous - but I got a couple of drops of solution on the wood and it stained.
Time to test our solution!
How cool is that?!
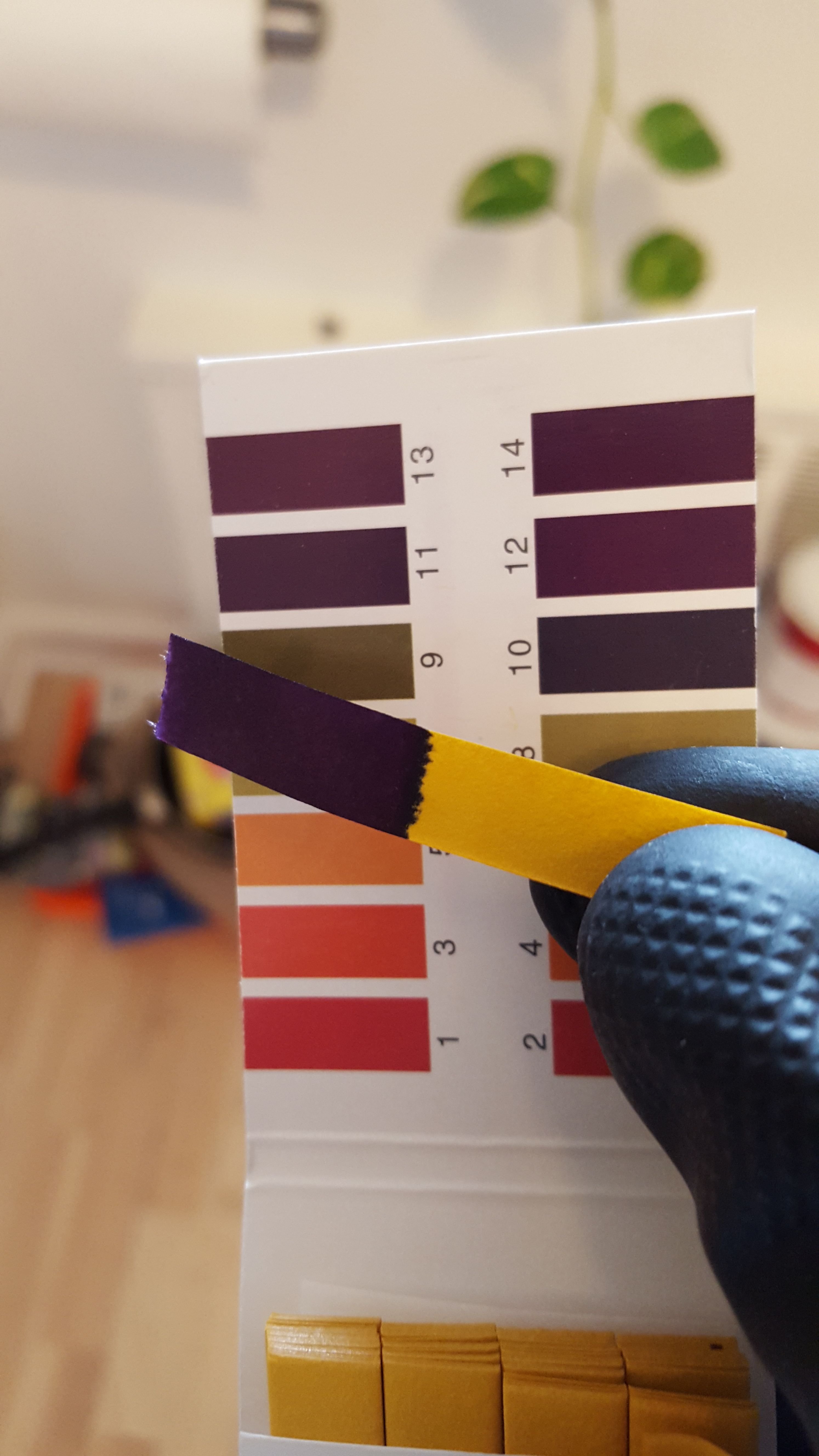
For those of you less than familiar with PH strips, take a look at the color comparison to the left.
Whereas the PH of the original distilled water was squarely in the middle of the PH spectrum, the PH of our new KOH solution is squarely on the base side of the spectrum, which is exactly where we want it. Now we know that our solution should work as intended. Over time, the solution will become less effective, so every once in awhile I will need to retest its PH to see if we need a new batch.

When filling two eye drop looking bottles with dangerous chemicals, labeling is important!
In this case, the bottle on the right is the KOH solution. Because my measurements were less than perfect, I labeled the bottle %4-5 percent, identified the contents and marked the creation date.
On the left is just a bottle filled with ammonium hydroxide, also known as Ammonia, which I bought at the store for less than $2. That was super simple and just required a funnel, so I didn't document the "process," such as it was.
This may not seem like much of a big step to some, but for certain genuses and species of mushroom, the addition of these two reagents into the pool of identifying characteristics can be very important. Several mushrooms reveal themselves, in part, through their reaction to KOH - including species in the genus Agaricus, Cortinarius, Gymnopilus, Leccinum and Russula, to name only a few. In the study and identification of boletes, the use of ammonium hydroxide can sometimes provide dispositive identifying markers.
Additionally, both KOH and NH4OH can sometimes be used in microscopy to assist in spore traits. We are still a ways away from my getting in depth on my microscopy, with the quality of my microscope being the primary chokepoint. However, in the future - perhaps come spring when the new mushroom season begins again - I may use my accrued steem to purchase a better microscope.
However you look at it, adding KOH and NH4OH to the mix increases the complexity and accuracy of my amateur study of fungi. It may not be the flashiest addition, but I'm pretty excited about it nonetheless!
THIS POST IS NOT INTENDED FOR FORAGING PURPOSES AND TO USE IT FOR THOSE PURPOSES WOULD BE DANGEROUS. DO NOT HUNT WILD MUSHROOMS WITHOUT RELYING ON A COMBINATION OF PROFESSIONAL FIELD GUIDES, IN PERSON PROFESSIONAL GUIDANCE, OR IN PERSON GUIDANCE BY SOMEONE TRUSTWORTHY WHO HAS COPIOUS LOCAL, SPECIALIZED MUSHROOM HUNTING EXPERIENCE. FAILURE TO DO SO CAN RESULT IN GRIEVOUS PERSONAL HARM OR DEATH.
Photos Are My Own Except for picture of silver:
[2]By Kuebi = Armin Kübelbeck Own work CC BY-SA 3.0, via Wikimedia Commons
Information Sources:
[1]Kuo on Mushroom Reagents
[2]Wikipedia on Chemical Testing Mushrooms
[3]The British Mycological Society On Reagents
[4]Wikipedia on KOH

