Today I started continuing my work on a project that may result in new filters to rid our water of estrogenic compounds. Today also marks the beginning of a new series of posts. I will try to report every day about work that I have done in the lab. With this I am hoping to get people a better understanding of what being a laboratory scientist looks like.
The science of today, is the technology of tomorrow.
Edward Teller
Preparations
Working in a laboratory encompasses a lot of cool things. However, the first steps of starting a new project are usually pretty unsensational. I am currently working in surface chemistry, which is a very precise branch of chemistry. The goal of my work is to fix an organic compound known as MUAM onto gold substrates. The gold substrates are 1 inch by 1 inch large glass slides, covered with a few nanometers of gold. They are very fragile and need to be handled with a lot of care. Another problem is that any organic compound can easily adhere to the gold surfaces, even organic compounds found in the air. In order to prevent contamination, the first task I had to perform was to clean lots of glassware.
After rinsing all the glassware I needed with superfiltered water (you know, water usually conducts electricity? This water is filtered so much that it acts as a resistor, alas does not conduct electricity at all!), I submerged it in a Helmanex bath. Helmanex is a solution that is made for cleaning glassware and other materials, and is something you definitely do not want to have on your hands. All of this I have done yesterday. Today, I had to take the glassware I was going to use from the Helmanex solution, and rinse it again with superfiltered water.
I also had to clean the metalware I was going to use, which included a pair of tweezers. The tweezers were corroded, and in order to clean them, I submerged them in a solution of 6M Nitric Acid (very concentrated, very corrosive).

The tweezers were very dirty (lots of rust), and the acid took really good care of removing the rust. Turns out, these tweezers were almost entirely rust.

Now lets move on to some cooler things.
What Piranhas are doing in my Lab
Piranha solution is a very nasty mix of sulfuric acid and hydrogen peroxide. Sulfuric acid by itself is very vicious, but mixed with hydrogen peroxide (commonly found in hair bleach), you get something really dangerous. The piranha solution I made was 70% sulfuric acid and 30% hydrogen peroxide. You can measure the needed amounts out very easily using a measuring cylinder, yet, never just dump the two components together. When mixed incorrectly, meaning too fast, piranha solution can bubble over the container you use (potentially causing harm by corosion) or explode, with a pressure wave and glass splinters. You do not want to have an acid bomb explode right next to you. I was actually very proud that my supervisor allowed me to prepare the piranha solution (students are usually not allowed to). Once that was set up, I practiced cleaning the gold substrates by using a simple and cheap glass cover slip. I was wearing thick rubber gloves and very pointy tweezers, which makes handling of the slips very difficult.
A friend of mine has just recently tried to perform the same project, but after breaking 3 gold substrates (each little slide is 50 Dollar), our supervisor decided to cancel this project for a few weeks. However, I managed it well and decided to clean the gold substrates. They were immersed into piranha solution for 3 minutes, and then placed into superfiltered water until further use.

As you can see I placed everything on wet paper towels. That is necessary to prevent any drops of piranha solution from coroding the working surface. Dry paper towels would have caught fire when getting in contact with piranha solution. As I mentioned above, piranha solution is vicious.
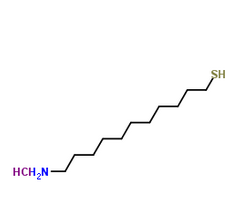
MUAM
MUAM is also known as 11-Amino-1-undecanethiol. It has two reactive groups at both of its ends. The thiol (sulfur hydrogen group), binds to the gold in a way we do not know yet. The amino group is later used for adding another substance. I will talk about this later. The MUAM is supposed to cover the entire gold surface.
Source
To fix MUAM to the gold surface, I prepared a 0.1 mili molar solution of MUAM in ethanol (drinking alcohol). To make 50 mililiter of MUAM I weighed out 0.0013 grams of MUAM using an analytical balance. The balance is very sensitive, and weight measurements are influenced by how far away from the balance you stand, airstreams, and water in the air adhering to your sample.
When the solution was set up, I had to move some of the gold substrates into the solution relatively quickly. Once the substrates were submerged, I sealed the containers with something called parafilm, which is a stretchy foil. I then covered the container with aluminum foil, labelled it as my samples and placed them in a fridge. The same happened to the blank substrates. Blank and samples are the same, only that the blanks are immersed in superfiltered water, instead of MUAM.
This is all the work I have done today. I was working from 9am until 3pm, with a 2 hour lunch break. Tomorrow I will set up equipment to perform Infra Red measurements, that will help me to demonstrate that MUAM formed a layer on the gold surface.
Thank you very much for reading my post! I really enjoy my work in the laboratory, however, I do understand that this is not for everyone.
If you liked this post, please upvote it and check our my channel. I am trying to give you a look behind the curtains.
Cheers @lesshorrible!


