Good day Steemians and welcome to my blog
Today article is about techniques involved in isolating microbes from the air
Isolation is often essential for taxonomic and experimental work on micro-organisms.
In the microbiological sense, it is the process of separating a single species of microorganism from its natural habitat and growing it by itself, without interference from other organisms, on a sterile substratum, i.e. in pure culture
Methods of isolating micro-organisms from a natural environment, such as soil, litter, air, or water, are numerous
There are two basic techniques of isolating microorganisms in the air
- Passive method
- Active method
Passive methods usually involve settle plates (sedimentation) whereas Active methods include impaction and impingement devices.
The volume of air for sample collection depends on the device being used and on the anticipated concentration of the bioaerosol.
Where low concentrations of microbial contaminants are expected, e.g. clean rooms, food production and operating theatres, impaction methods are generally chosen.
In highly contaminated environments, impaction techniques may 'oversample' even over short timescales and impingement or filter samples are more appropriate.
With the strict adherence to manufacturer's flow rates, sampling periods, culture media used and device placement, most techniques should yield comparable results, which are normally expressed in cfu/m³ (CFU= colony forming unit).
PASSIVE METHODS
Sedimentation method
This ‘Settling Plate Technique’ based on this approach is the simplest and is often used by air microbiologists.
The principle behind this method is that the microbes carrying particles are allowed to settle onto the medium for a given period of time and incubated at the required temperature. A count of colonies formed shows the number of settled bacteria containing particles. In this method Petri dishes containing an agar medium of known surface area are selected so that the agar surface is dry without any moisture.
Choice of the medium depends upon the kind of microorganisms to be enumerated. For an overall count of pathogenic, commensal and saprophytic bacteria in air blood agar can be used. For detecting a particular pathogen which may be present in only small numbers, an appropriate selective medium may be used. Malt extract agar can be used for molds. The plates are labeled appropriately about the place and time of sampling, duration of exposure etc. Then the plates are uncovered in the selected position for the required period of time.
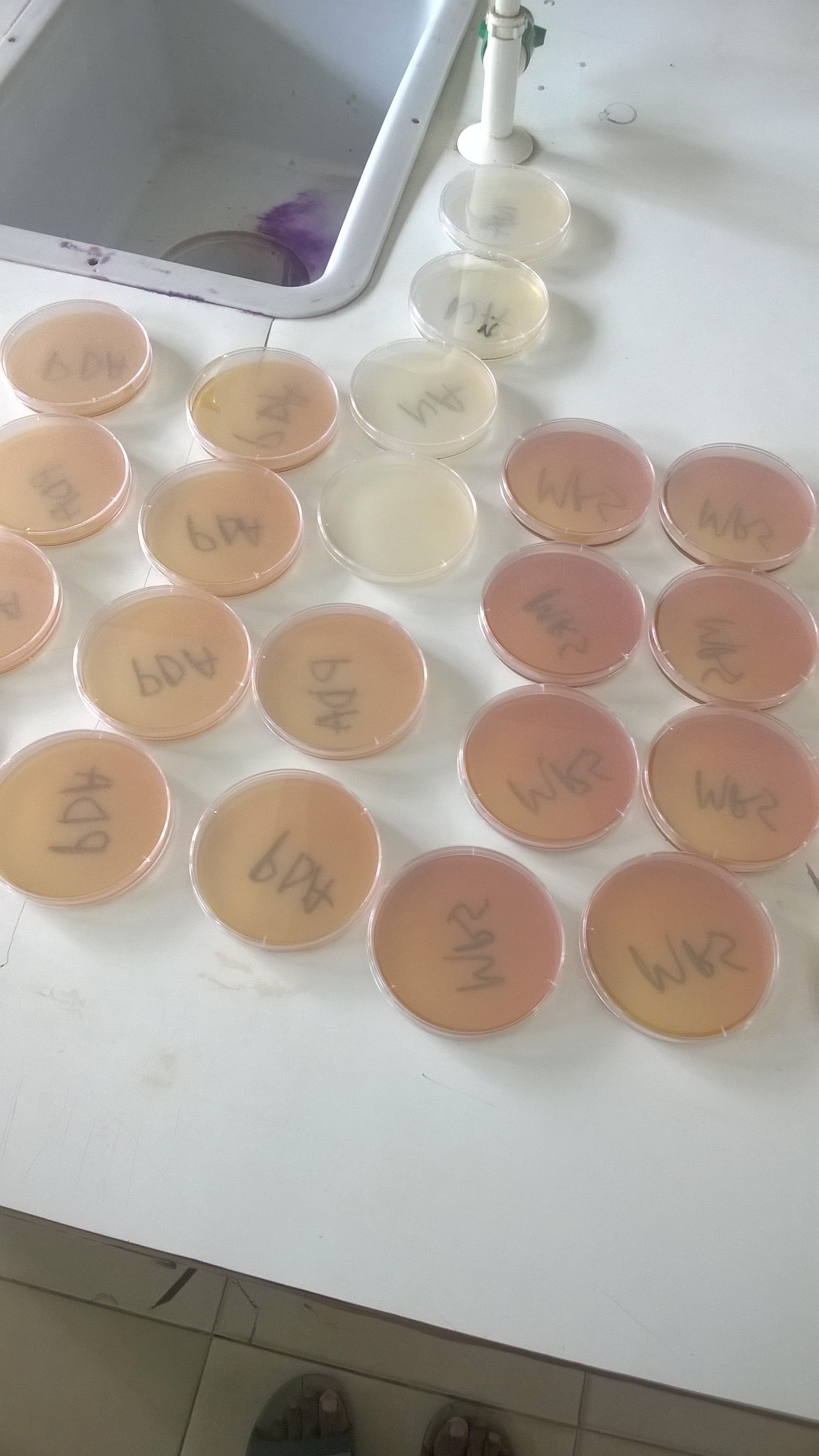
A Petri dish containing agar medium is kept covered and, at the time of sampling, the cover is removed from the Petri dish so that the agar surfaces is exposed to air for a few minutes. The Petri dish is now incubated.

One can see a certain number of colonies developing on agar medium. Each colony represents a particle carrying microorganisms which has fallen on the agar surface. The optimal duration of exposure should give a significant and readily countable number of well isolated colonies, for example about 30-100 colonies.
Usually it depends on the dustiness of air being sampled. In occupied rooms and hospital wards the time would generally be between 10 to 60 m. During sampling it is better to keep the plates about 1 meter above the ground. Immediately after exposure for the given period of time, the plates are closed with the lids. Then the plates are incubated for 24hrs at 37°C for aerobic bacteria and for 3 days at 22°C for saprophytic bacteria. For molds incubation temperature varies from 10-50°C for 1-2 weeks.
After incubation the colonies on each plate are counted and recorded as the number of bacteria carrying particles settling on a given area in a given period of time.
The use of settle plates is not recommended when sampling air for fungal spores, because single spores can remain suspended in air indefinitely. Settle plates have been used mainly to sample for particulates and bacteria either in research studies or during epidemiologic investigations.
Results of sedimentation sampling are typically expressed as numbers of viable particles or viable bacteria per unit area per the duration of sampling time (i.e. CFU/area/time); this method cannot quantify the volume of air sampled. Because the survival of microorganisms during air sampling is inversely proportional to the velocity at which the air is taken into the sampler, one advantage of using a settle plate is its reliance on gravity to bring organisms and particles into contact with its surface, thus enhancing the potential for optimal survival of collected organisms.
This process, however, takes several hours to complete and may be impractical for some situations.
All pictures were taken with my windows phone
Stay tuned for the next part of these interesting topic
Kindly follow, upvote, resteem, and comment if you like this post
