During World War II jewel bearings were one of the products restricted by the United States government War Production Board as critical to the war effort.
World War II lasted about 6 years.
World War II changed the political alignment and social structure of the globe.
- The United Nations (UN) was established to foster international co-operation and prevent future conflicts.
Did it? Or did it become a far more powerful and controlling entity?
You decide!
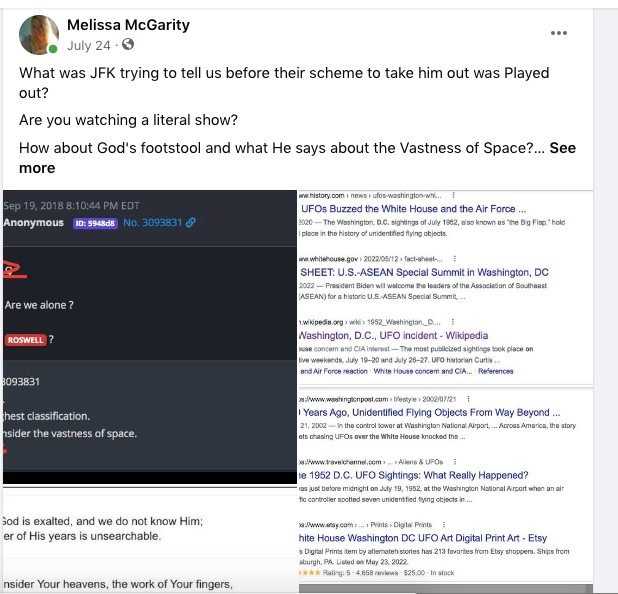
History of the United Nations and what they have really been up to.
Hear an official there say there are a Compound while operating in a State in the United States.
Do you find this interesting?
They gained this invite through a university that supports the UN. Do you find that interesting?
with the victorious great powers—China, France, the Soviet Union, the United Kingdom, and the United States—becoming the permanent members of its Security Council.
The Soviet Union and the United States emerged as rival superpowers, setting the stage for the nearly half-century-long Cold War. In the wake of European devastation, the influence of its great powers waned, triggering the decolonisation of Africa and Asia. Most countries whose industries had been damaged moved towards economic recovery and expansion. Political and economic integration, especially in Europe, began as an effort to forestall future hostilities, end pre-war enmities and forge a sense of common identity.
Is time linear as people think or does it occur in a manner people are literally Blinded to?
God said, "Let there be LIGHT!"
Sound created light? He said it and it WAS!
Now God IS the word. . .how do we know/
John 1:1 In the beginning was the Word and the Word was with God and the Word WAS God!
So Sound Manifests LIGHT right?
Therefore. . .sound manifests toroidal fields.
Those toroidal fields create magnetic fields
Moving magnetic fields create electricity.
Since sound creates the magnetic. . .everything must be magnetic in some sort of way.
If it's not, there's a very specific reason why it's not magnetic.
Even water is magnetic as are
Graphite
Aluminum
Glass
Oxygen
Tesla didn't believe in the programming they put in place for humanity.
He did not believe in the existence of an electron as pictured by science.
Was an agenda being furthered by mainstream universities and by those trying to control humanity? If so Why?
Did they want to remain in control while profiting? You decide!
Einstein also pointed to the problem the mainstream scientists [accepted by the masses] continued to promote and push as truth, though they couldn't explain the glaring problem with that narrative.
He claimed people of the world were being fooled by the non existing electron.
So why is it being forced down everyone's else's throats and in esteemed educational institutions.
Why do the controllers and gatekeepers of information try to censor and silence those who are critical thinkers and test their narrative? Is it because the Truth threatens them?
Along another Wavelength. . . in keeping time, consider Ingersoll watches.
Ingersoll was set up by two brothers – Robert and Charles – in 1882. It took the Ingersoll siblings a little bit of time to work out exactly what they wanted to sell but eventually landed on retailing pocket watches at an affordable price. By 1896 they introduced the cheapest watch available on the market – the Yankee or ‘dollar watch’ which, as you may have worked out cost $1. A few years later they set up shop in Britain, under a subsidiary company, and began selling the Crown pocket watch for a similarly affordable five shillings.
These well-produced watches flew off the shelves. By the mid-1910s Ingersoll added an innovation to their range: ‘Radiolite’ glow in the dark dials and hands made from a paint utilising radium salts. That radium glowed in the dark had first been shown by the Curies in the late 1890s, and it was soon realised that if you added something sticky to the salts you could turn it into paint.
At first, it wasn’t understood how radium salts glowed: but it was later confirmed that the radiation emanating from the salts agitated the nitrogen which is naturally present in the air. This vibration causes a buzz of energy which is perceptible as a very faint shimmer of light.
This effect meant that radium paint glowed all by itself and – unlike other glow in the dark paints available at the time – was permanent and didn’t need to be ‘charged up’ by an outside substance or by light.

An advert with a poem from 1917 explains in rhyme how useful Radiolite watches could be:
“Come boys, let’s get the matter right.
What is a truly RADIOLITE?
It is no RADIOLITE at all
Unless it is an INGERSOLL.
A boy may have a watch that goes,
But it’s a half-watch if it shows
The time of day, but skips the night
Unless you fuss to get a light.
You want the time and you should know it;
The watch to have should always show it.
Old Daddy Time has lots of laughs
At watches that tell time by halves.
The RADIOLITE- the INGERSOLL make
Is always brightly wide awake.
It shines at night, it never sleeps
As faithful watch and time it keeps.
Its figures and its hands are bright
And plain by day, and clear by night.
How does this friendly brightness come?
This light is made by RADIUM.
It isn’t phosphorescent paint
That shines a while and then grows faint.
It’s RADIUM, that magic bright
That makes your trusty RADIOLITE.
Yes, INGERSOLL’S the RADIOLITE,
Just hold this in your memory tight.
You’re big enough to do the rest.
Go buy the model you like best.
There, boys, I think you have it right;
You know who makes the RADIOLITE.
It is no RADIOLITE at all
Unless it is an INGERSOLL.
Ingersoll’s main advertising target was service personnel – or rather the family of service personnel – a market which grew massively during the First World War.
Watches, in the form of pocket watches, had always been an important part of an officer’s kit but their usefulness was brought into question by new methods of fighting. A pocket watch was impractical to use on horseback, or in the open cockpits of early aircraft or in the cramped conditions of the trenches. Wristwatches were an effective solution but didn’t solve the problem of being able to see one’s watch in the dark: a vital necessity for timed and synchronised manoeuvres.
Striking a match was dangerous because it might be seen by an enemy sniper. Watches with self-luminous dials therefore quickly became an essential safety element: a must-have gadget.
Because they were so essential for safety, glow-in-the-dark wrist- watches became a popular gift to send to loved ones in service. The adverts for these were pushed particularly hard at Christmastime: ‘Not only will a reliable watch afford him good service, but it will prove a real companion and friend.’
After the war had ended glow in the dark wristwatches were hugely fashionable and this is where our object comes in. I don’t have any particular date for it but stylistically we can date to the 1920s.
This was a decade that bought some difficulty to Ingersoll. The US company went bankrupt in 1921 and the UK based company was sold off and eventually wound up in 1934. I actually don’t know exactly what happened to the company – but the end of the war, massive downturn in the economy, and a backlash to radium watches following the much publicised deaths of the women whose job it was to paint them would have all contributed.
The US company was bought by the Waterbury Clock Company and became the United States Time Corporation then Timex. Whatever the company was called they continued to utilise the Ingersoll name and even today you can still buy an Ingersoll watch. It is, however, worth noting that their ‘Radiolite’ range no longer contains radium!














Ingersoll Lockwood's Uncle Ralph
Ralph Ingersoll Lockwood (1798 Greenwich - 1855 New York City) was an American political writer, lawyer and novelist. Lockwood was one of 136 signatories to an 1838 petition to Congress on the matter of copyright and intellectual property.[3] He also wrote under the pseudonym "Mr. Smith".Lockwood's nephew, Ingersoll Lockwood, was also a lawyer and writer.
He wrote these novels,
The Insurgents. Philadelphia: Carey, Lea and Blanchard (1835)[5]
Rosine Laval
These were his political writings,
An Address to the Republicans and People of New-York, Pennsylvania and Virginia, Upon the State of Presidential Parties
Find it digitized here,
https://unz.com/print/LockwoodRalph-1835/




prudence
[ prood-ns ]SHOW IPA
noun
the quality or fact of being prudent, or wise in practical affairs, as by providing for the future.
caution with regard to practical matters; discretion.
regard for one's own interests.
provident care in the management of resources; economy; frugality.



Ingersoll's Lecture on Thomas Paine—Delivered in Central Music Hall,
Chicago, January 29, 1880 (From the Chicago Times, Verbatim Report)
Interesting as same period of the above political novel by Ingersoll Lockwood's uncle Ralph?
Remember. . .for Ingersoll Lockwood's family heritage. . .
Family History
Ingersoll was born to Munson Ingersoll and Sarah Lewis (née Smith) Lockwood.
Munson,
like his brothers was a lawyer.
achieved prominence during his military service and civic activism.
He was a general in the New York State Militia and commandant of its 7th Brigade.
one of the founders of Ossining's first bank and Dale Cemetery
served as the Warden of Sing Sing prison from 1850 to 1855.
Sing Sing Correctional Facility, formerly Ossining Correctional Facility
a maximum-security prison operated by the New York State Department of Corrections and Community Supervision in the village of Ossining, New York.
Like his father and uncles, Ingersoll Lockwood trained as a lawyer, although his first position was as a diplomat. In 1862 he was appointed Consul to the Kingdom of Hanover by Abraham Lincoln. At the time he was the youngest member of the U.S. consular force and served in that post for four years. On his return he established a legal practice in New York City with his older brother Henry.
See more inside of here,
Ingersoll Lockwood
@artistiquejewels/ingersoll-lockwood
From inside the above article you will find,
Lockwood spent his retirement years as a recluse in Saratoga Springs, New York where he published his last book, a collection of poetry entitled In Varying Mood, or, Jetsam, Flotsam and Ligan in 1912. It opens with juxtaposed photographs of Lockwood at age 35 and at age 70. In the preface, he wrote:
The end has almost come. I'm only waiting for the signal to push off and begin my voyage to the Isles of the Blest in the far Western Seas. I was troubled in my mind at first, for my little bark, staunch though it may be, sat too deep in the water. It was overladen with conceits that wouldn't be current and merchandise that wouldn't be saleable in the Isles of the Blest. Overboard with it! Now that I have lightened ship I feel better.
Lockwood died in Saratoga Springs five years later, in 1918, at the age of 77. He had no children or surviving relatives.
Going back to the time keeper Lockwood brothers. . .

According to TU Delft,
QuTech creates a time crystal
n the crystals we encounter in our daily lives, such as diamonds or table salt, the atoms spontaneously form a stable repeating pattern in space. Can something similar happen in time?
The idea of a time crystal was first proposed in 2012 by Frank Wilczek, a physicist and Nobel laureate. The ensuing debate led to the prediction that time crystals can form in periodically driven interacting quantum systems. The system is then locked in a stable pattern that oscillates between two discrete states. In such a discrete time crystal, disorder in the internal interactions can prevent the system from reaching thermal equilibrium or heating up. In theory, it can oscillate forever without any net absorption of energy.
“We set out to build a discrete time crystal using one of our quantum processors based on spins in diamond,” says Joe Randall from QuTech, a collaboration between Delft University of Technology and TNO. “These spins form extremely well-isolated and individually controlled quantum bits that we can program to emulate other physical systems.”
Working together with collaborators from UC Berkeley and Element Six,
**the team used nine quantum bits and manipulated them in just the right way to satisfy the theoretical criteria to form a time crystal. **
When tuned into the right parameter regime, the spins are locked together into a periodically inverting pattern that is robust against perturbations of the system.
Importantly, the team then showed that the time crystal formed when starting from all sorts of initial states.
**“This observation of a robust response for all starting states was really the smoking gun for the time crystal being stabilized by disorder in its internal interactions,” **says Conor Bradley, PhD student at QuTech. “It is what distinguishes our results from previous investigations.”
perturbation - (physics) a secondary influence on a system that causes it to deviate slightly. natural philosophy, physics - the science of matter and energy and their interactions; "his favorite subject was physics" influence - the effect of one thing (or person) on another; "the influence of mechanical action"
The time crystal created by the team lives remarkably long: it lasts up to about 800 periods, or about 8 seconds. “While a perfectly isolated time crystal can, in principle, live forever, any real experimental implementation will decay due to interactions with the environment,” says Randall. “Further extending the lifetime is the next frontier.”
Just one month after the researchers went public with their data, a team from Google, Stanford and others reported the realization of a discrete time crystal using a superconducting quantum computer. “It is extremely exciting that multiple experimental breakthroughs are happening simultaneously,” says Tim Taminiau, lead investigator at QuTech. “All these different platforms complement each other.
The Google experiment uses two times more qubits, our time crystal lives about ten times longer.” Indeed, theory collaborator Norman Yao from UC Berkeley believes that this is just the beginning, “A time crystal is perhaps the simplest example of a non-equilibrium phase of matter. The QuTech system is perfectly poised to explore other out-of-equilibrium phenomena including, for example, Floquet topological phases.”
Many open questions remain. Are there practical applications for time crystals? What will be observed in higher spatial dimensions? And, in general, how do driven quantum systems equilibrate? The spin defects in solids used by the team provide a flexible platform for experimentally studying these important open questions in statistical physics. “The ability to isolate the spins from their environment while still being able to control their interactions offers an amazing opportunity to study how information is preserved or lost," says Francisco Machado, one of the collaborators from UC Berkeley. “It will be fascinating to see what comes next.”
The publication in Science is the result of a collaboration of QuTech, University of California Berkeley and Element Six, which grew the ultrapure diamonds used in the research.
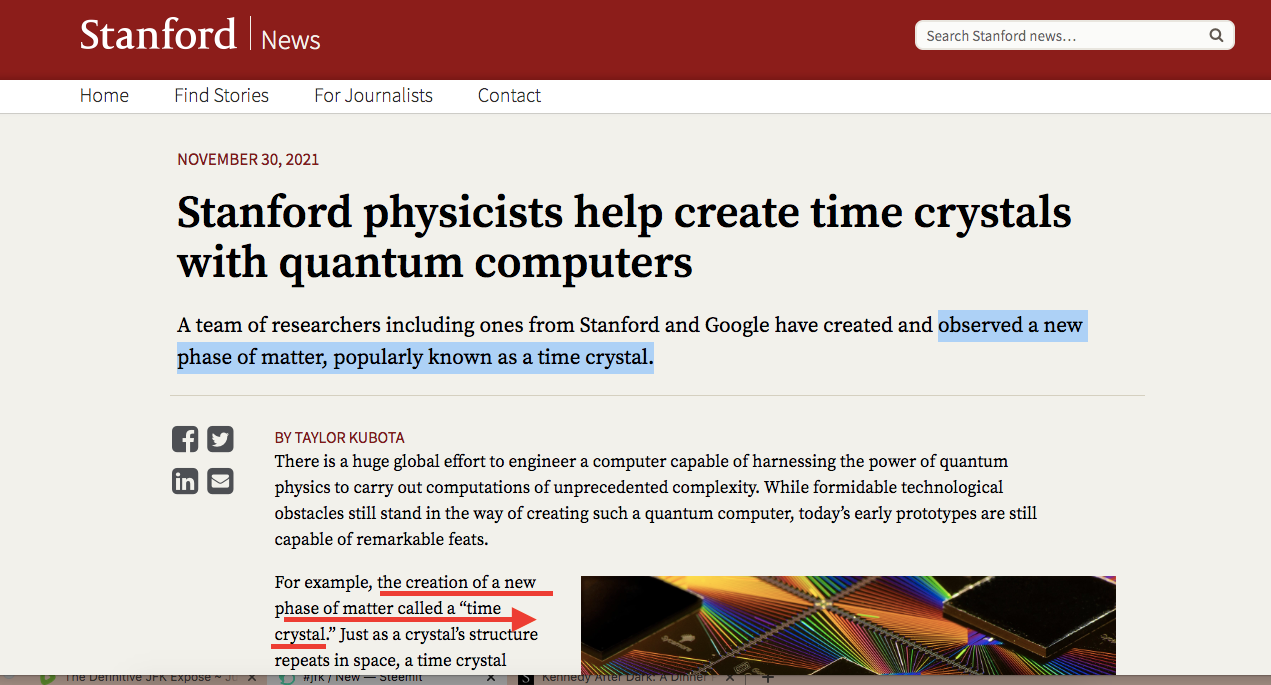

According to Stanford News,
There is a huge global effort to engineer a computer capable of harnessing the power of quantum physics to carry out computations of unprecedented complexity. While formidable technological obstacles still stand in the way of creating such a quantum computer, today’s early prototypes are still capable of remarkable feats.
The Google Sycamore chip used in the creation of a time crystal. (Image credit: Google Quantum AI)
For example, the creation of a new phase of matter called a “time crystal.” Just as a crystal’s structure repeats in space, a time crystal repeats in time and, importantly, does so infinitely and without any further input of energy – like a clock that runs forever without any batteries. The quest to realize this phase of matter has been a longstanding challenge in theory and experiment – one that has now finally come to fruition.
In research published Nov. 30 in Nature, a team of scientists from Stanford University, Google Quantum AI, the Max Planck Institute for Physics of Complex Systems and Oxford University detail their creation of a time crystal using Google’s Sycamore quantum computing hardware.
“The big picture is that we are taking the devices that are meant to be the quantum computers of the future and thinking of them as complex quantum systems in their own right,” said Matteo Ippoliti, a postdoctoral scholar at Stanford and co-lead author of the work. “Instead of computation, we’re putting the computer to work as a new experimental platform to realize and detect new phases of matter.”
The basic ingredients to make this time crystal are as follows: The physics equivalent of a fruit fly and something to give it a kick. The fruit fly of physics is the Ising model, a longstanding tool for understanding various physical phenomena – including phase transitions and magnetism – which consists of a lattice where each site is occupied by a particle that can be in two states, represented as a spin up or down.
During her graduate school years, Khemani, her doctoral advisor Shivaji Sondhi, then at Princeton University, and Achilleas Lazarides and Roderich Moessner at the Max Planck Institute for Physics of Complex Systems stumbled upon this recipe for making time crystals unintentionally. They were studying non-equilibrium many-body localized systems – systems where the particles get “stuck” in the state in which they started and can never relax to an equilibrium state.
They were interested in exploring phases that might develop in such systems when they are periodically “kicked” by a laser. Not only did they manage to find stable non-equilibrium phases, they found one where the spins of the particles flipped between patterns that repeat in time forever, at a period twice that of the driving period of the laser, thus making a time crystal.
The periodic kick of the laser establishes a specific rhythm to the dynamics. Normally the “dance” of the spins should sync up with this rhythm, but in a time crystal it doesn’t. Instead, the spins flip between two states, completing a cycle only after being kicked by the laser twice.
This means that the system’s “time translation symmetry” is broken. Symmetries play a fundamental role in physics, and they are often broken – explaining the origins of regular crystals, magnets and many other phenomena; however, time translation symmetry stands out because unlike other symmetries, it can’t be broken in equilibrium.
The periodic kick is a loophole that makes time crystals possible.
The doubling of the oscillation period is unusual, but not unprecedented. And long-lived oscillations are also very common in the quantum dynamics of few-particle systems. What makes a time crystal unique is that it’s a system of millions of things that are showing this kind of concerted behavior without any energy coming in or leaking out.
“It’s a completely robust phase of matter, where you’re not fine-tuning parameters or states but your system is still quantum,” said Sondhi, professor of physics at Oxford and co-author of the paper. “There’s no feed of energy, there’s no drain of energy, and it keeps going forever and it involves many strongly interacting particles.”
While this may sound suspiciously close to a “perpetual motion machine,” a closer look reveals that time crystals don’t break any laws of physics. Entropy – a measure of disorder in the system – remains stationary over time, marginally satisfying the second law of thermodynamics by not decreasing.
For Khemani and her collaborators, the final step to time crystal success was working with a team at Google Quantum AI. Together, this group used Google’s Sycamore quantum computing hardware to program 20 “spins” using the quantum version of a classical computer’s bits of information, known as qubits.
Revealing just how intense the interest in time crystals currently is, another time crystal was published in Science this month. That crystal was created using qubits within a diamond by researchers at Delft University of Technology in the Netherlands.
https://news.stanford.edu/2021/11/30/time-crystal-quantum-computer/
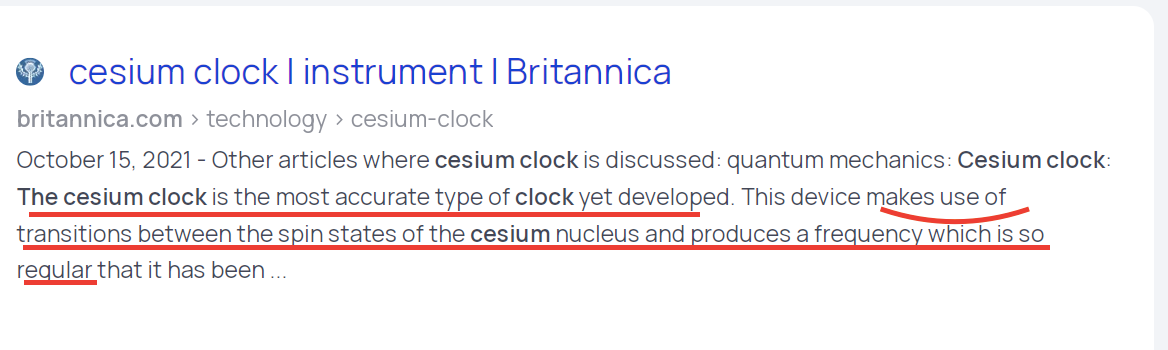

The cesium clock is the most accurate type of clock yet developed. This device makes use of transitions between the spin states of the cesium nucleus and produces a frequency which is so regular that it has been adopted for establishing the time standard. Like electrons, many atomic nuclei have spin. The spin of these nuclei produces a set of small effects in the spectra, known as hyperfine structure.
From the following e-book The Britannica Guide to Relativity and Quantum Mechanics
The oft-referenced E = mc2 may perhaps be one of the world s most famous equations, but it actually represents only one aspect of the complex branch of physics known as relativity. Together, relativity and quantum mechanics explain both the most cosmic and most elementary relationships and processes of the universe. The profound place that relativity and quantum mechanics occupy in subverting longstanding notions about space, time, matter, and more and the brilliant individuals who advanced study in these fields are the subjects of this volume.
Remember. ..an isotope is. . .
each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and hence differ in relative atomic mass but not in chemical properties; in particular, a radioactive form of an element.
Remember all atoms in the periodic table are made from 3 kinds of particles,
Protons
Electrons
Neutrons
Protons and neutrons are said to live in the atom's center called the nucleus.
Protons carry positive electric charge
Neutrons are neutral
The light electrons buzz and speed around the outside of the nucleus which seem to create the shape of a fuzzy ball.
Electrons carry a negative electric charge.
Does a neutral object have an equal amount of positive and negative charges?
Positively charged. . .more positive charges
Negatively charged. . .more negative charges
Objects placed side by side that have different charges create an attractive force between the two.
But the same objects with the same charges placed side by side whether they are both positive or both negative. . .they will repel each other.
If you move an object up to a neutral object. . .it can Polarize it!
So it can move some of the charges to one end to create a similar charged object.
Remember, an electric charge can be either positive or negative.
In neutral objects. . .equal amount of positive and negative charges.
For instance, a charged object like a balloon that has received static will have more of a positive charge.
Now if it is a negatively charged object more of a negative charges inside.
Think of two neutral objects like two balloons that have not been charged.
What happens if you let them go? Nothing because charges are equally distributed.
Opposites attract so objects with different charges will attract and move together.
If you take a negatively charged object and place it near a neutral object. . .the negative charged object attracts the opposite charge in the neutral object [brings together the protons for an attraction].
If you rub a balloon on your hair or a sweater. . .the balloon picks up all those negative charges.
The balloon is no longer neutral, but negatively charged, leaving the sweater or hair positive; therefore the balloon will be attracted to the hair or sweater do to the opposing charges left.
Polarizing means it groups the positive charges and negative charges thus causing the attraction of the charged object to what once was a neutral object. So polarizing can lead to an attraction.
Two balloons charged from the sweater or hair will repel each other as like charged objects repel.
So the distribution of charges within an object tells us whether it will be positively or negatively charged.
Stuff around is not electrically charged most of the time, this means atoms are overall neutral.
This means the positive charge of the proton, must be exactly cancelled by the negative charge of an electron.
The number of electrons and protons must be the same.
Let's take a Hydrogen atom for example.
Atomic number 1
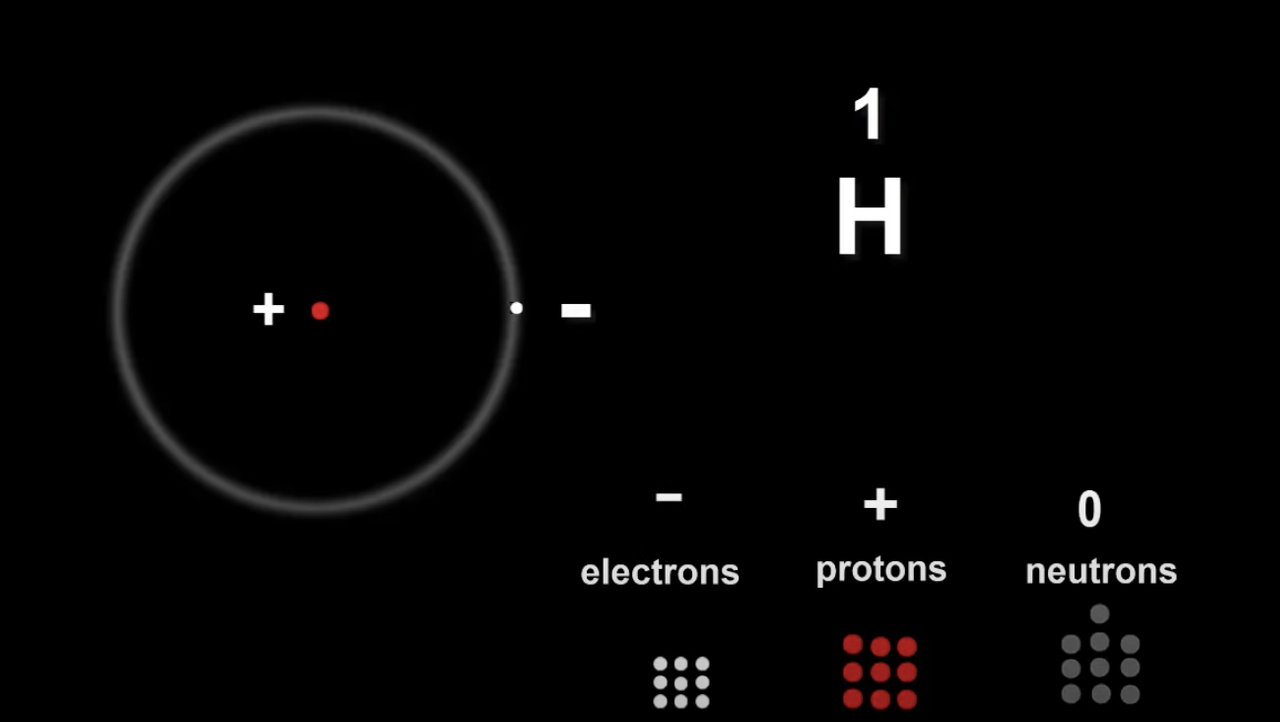
The number of electrons equals the number of protons and this is true for all atoms.
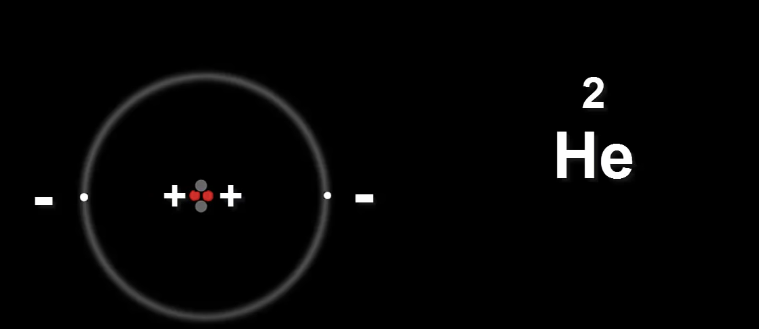
Now let's take Helium He

The two protons in the nucleus carry the same charge.
This is a problem because they will repel each other.
Meaning the nucleus will fly apart.
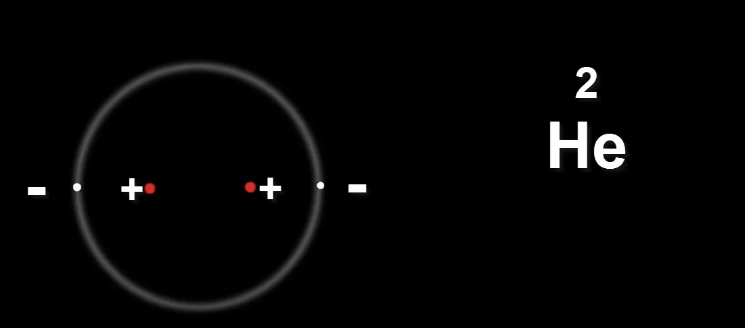
Neutrons provide the extra glue to hold the nucleus together.
There is an extra attractive force that belongs in the nucleus, this is called the Strong Nuclear Force, which works Only Between nuclear particles, and without it the nucleus would disintegrate.
Why doesn't a Hydrogen atom need a neutron?
It has only one proton in it's nucleus.
This means it won't feel any repulsive forces by nearby neutrons.
Hydrogen is the Only Atom that can do this.
All other atoms must have neutrons in their nucleus to keep it together.
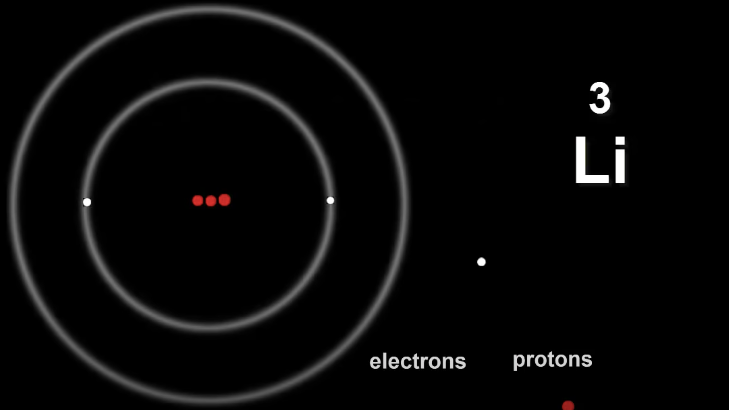
Let's look at Lithium Li

Electrons live in shells that are wrapped around the nucleus.
The first shell can take only two electrons before it becomes full.
This means Li has too many electrons to squeeze them into the first shell.
There are plenty of shells available so once first shell is filled, the third electron can go into the next shell.
The second shell being larger than the first can take up to 8 electrons.
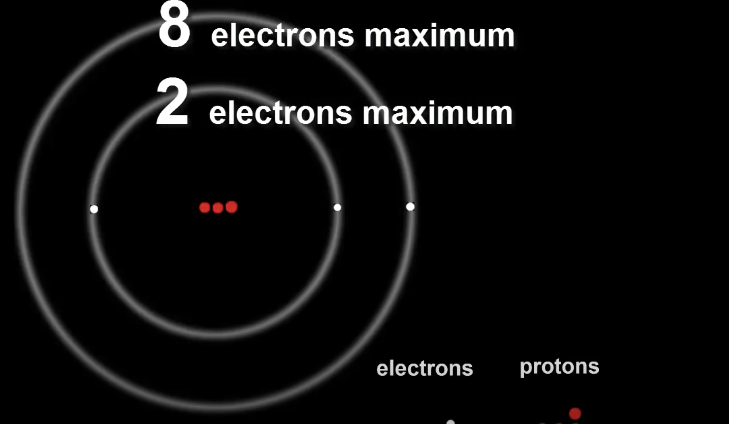
The number of electrons in each shell is very important as it effects the properties of the element and how it will behave.
The single electron in the outer shell of Lithium is what makes it a metal.
The number of electrons in each shell of an atom is called it's Electron Configuration.
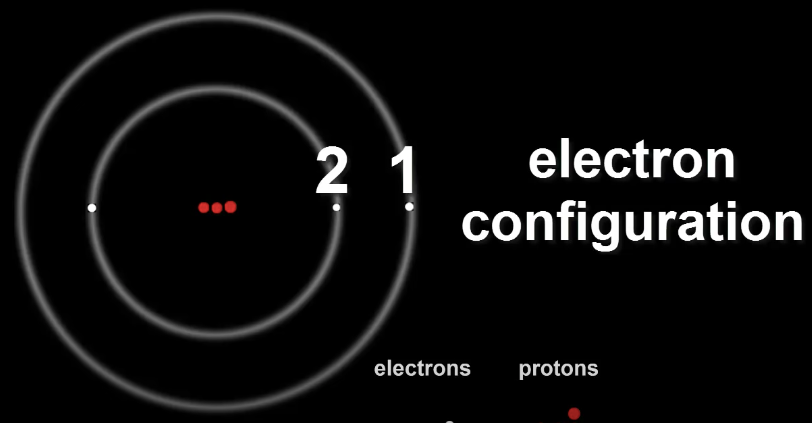
Lithium's configuration can be denoted as 2,1.
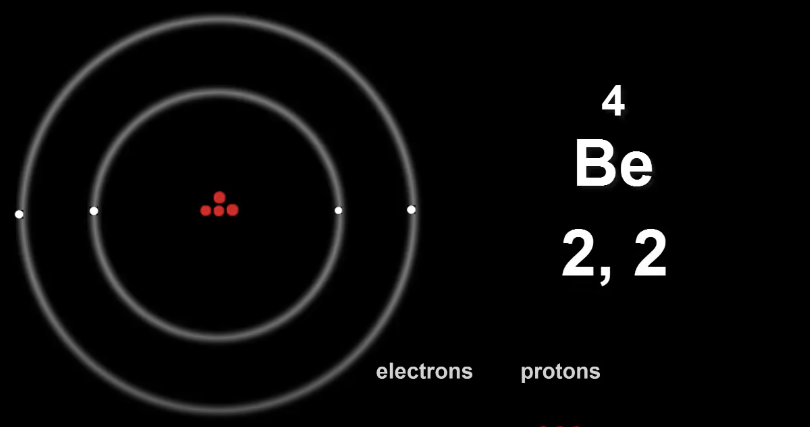
Here is Beryllium's electron configuration
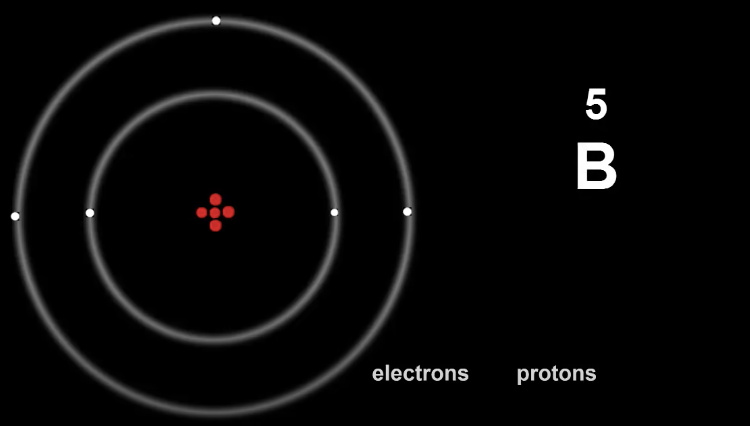
Here is Boron, with it's electron config being 2, 3
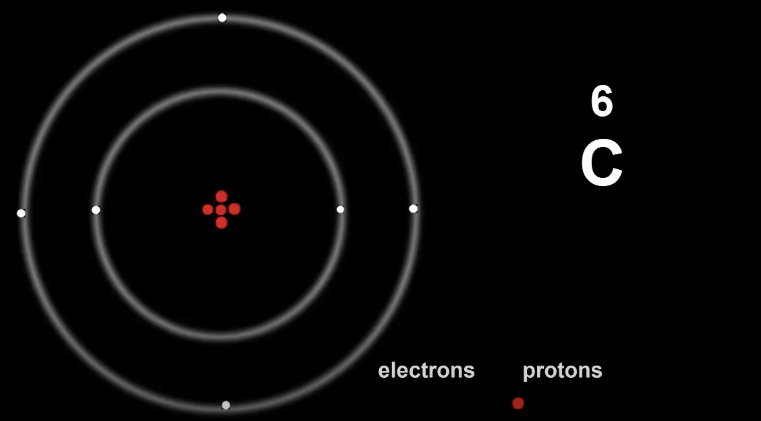
Here is Carbon, so 2, 4
When scientists discovered sub atomic particles, they realized the atomic number is also equal to the number of protons in the nucleus.
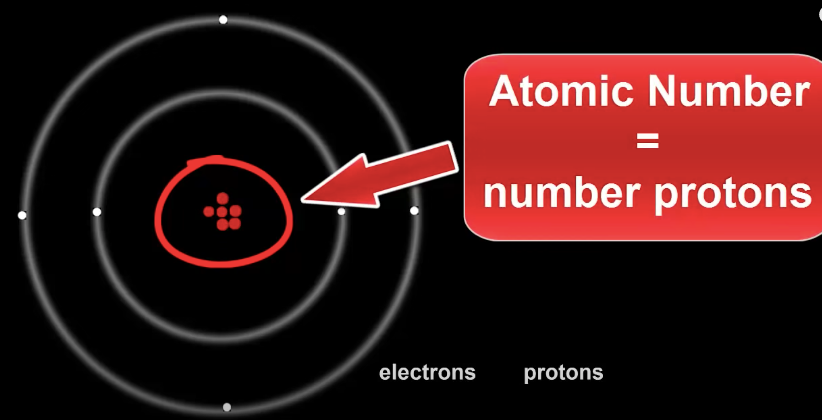
This means an Atom's identity only comes from how many protons are in it's nucleus.
Even though the electrons are to match the number of protons, they cannot be used for identity because they can sometimes be swept off the outside shell of an atom or an atom may grab an extra electron or more.
The number of electrons can vary at times.
Protons live in the nucleus which is locked away from the outside world, meaning their numbers generally do not vary.
Nitrogen is 7 with it's electron config being 2, 5
Oxygen is 8 with electron configuration of 2, 6
Fluorine is 9 electron config. . .2, 7
Neon is 10 ec is 2, 8
As the 2nd shell of an atom can only take up to 8 electrons, neon's 2nd shell is full.
This has important consequences. . .it makes it an un reactive noble gas.
Mass number equals the number protons plus number of neutrons.
Electron mass is negated as it is so tiny.
Number of neutrons stays the same, but neutrons cause the difference in mass.
Atoms of the same element with a different mass number are known as isotopes.
So same number of protons, but different number of neutrons.
Isotopes of elements occupy the same spot on the periodic table.
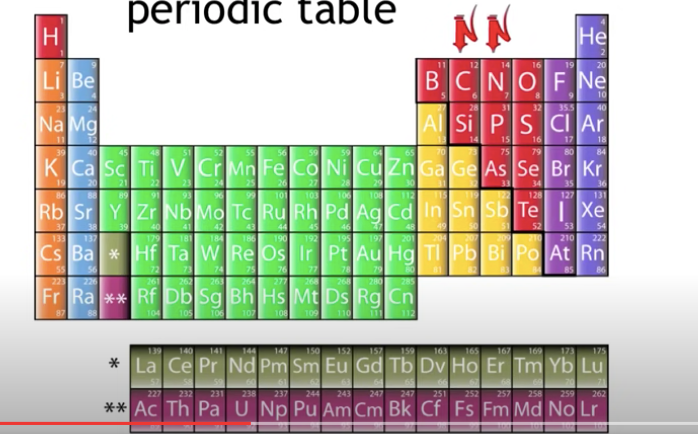
Mass number = protons + neutrons; therefore
Neutrons = Mass number - protons.
Isotopes only differ in their number of neutrons and mass.
Their chemical properties are exactly the same because neutrons have no effect on an element's chemical behavior.
Their chemical behavior is controlled by electrons.
Isotopes are essentially atoms of the same element with the same number of protons, but different number of neutrons.
They have different masses, but their chemical properties are exactly the same.
Many years ago radioactivity was seen to be a reservoir of health and vitality.
Why did it change?
Who made it change?
You have to remember what maintains the stability of an atom is the nucleus.
Inside of an atom it is sort of when you are about to sneeze and that odd feeling before and sometimes it stops and you don't sneeze.
An atom has the urge to expel the excess and regain stability.
The nucleons of positive protons and neutral neutrons are held together by the glue mentioned earlier. . .TheStrong Nuclear Force
It cancels out the repulsive electrostatic force of like charged protons and keeps the nucleus stable.
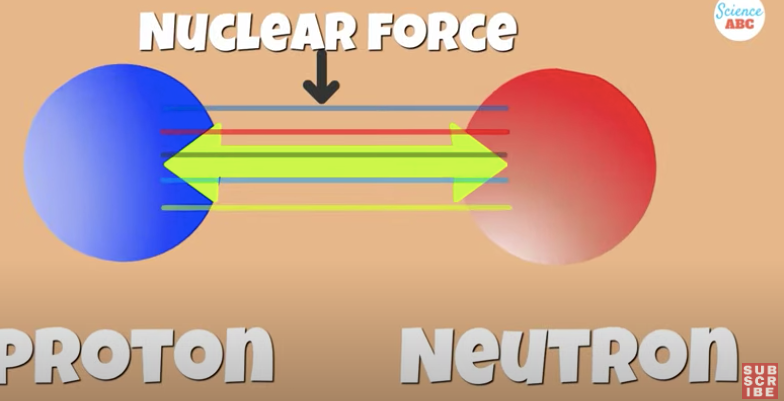
This nuclear force has a short range of action and is dependent on the ratio of protons and neutrons present in the nucleus.
You can see this balance start to waiver when the number of neutrons exceeds the number of protons.
A nucleus that exceeds the number of neutrons that can be held together comfortably by the Strong Nuclear Force will become more unstable.
These give rise to unstable isotopes of elements.
Just as your body through a series of contractions and expansions eventually expels the sneeze, the isotopes of elements fling out different particles or forms of energy to restore balance between the forces in their nucleus.
During this process of attaining stability, they change into a new nucleus.
The property of turning into something New to attain stability is called radioactivity.
The process by which it transforms is called Radioactive Decay.
A nucleus can undergo nuclear or radioactive decay through
Alpha
Beta
Gama Radiation
Sometimes a combination of all three.
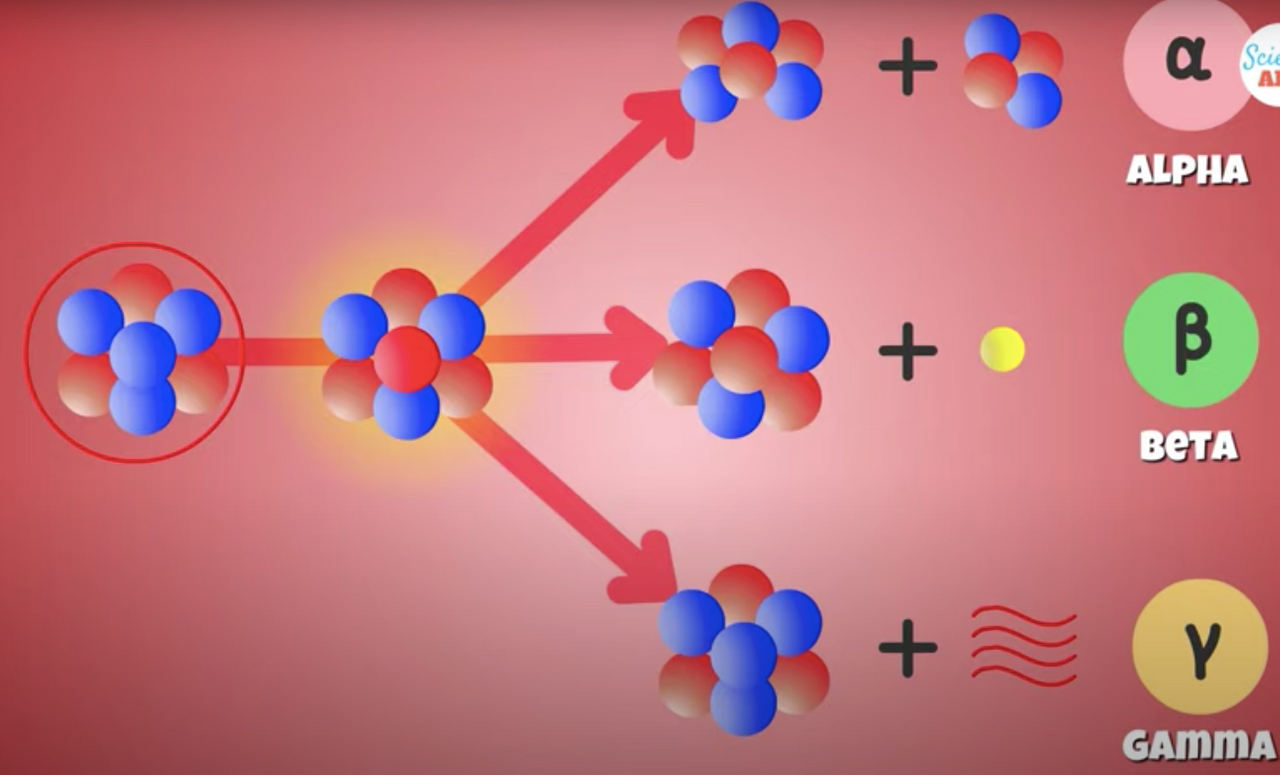
The** Alpha particles** are pretty heavy, so can only travel a few centimeters in the air.
They can easily be stopped by a sheet of paper or plastic.
Beta particles have higher energy and more penetrating power than Alpha particles, but are less ionizing in nature.
They can travel through the air, but can be stopped by a thin sheet of metal or even protective clothing.
The most energetic and fatal form of radioactive decay are Gamma Rays
A form of high energy light emitted by a nucleus that is left at a higher energy state after an Alpha and Beta decay process, but must still return to a more stable lower energy state.
A nucleus can undergo all of these decay stages simultaneously and transform into a stable form within seconds, years or even centuries.
This rate of decay is called the half-life of the radioactive substance.
This measures the time it takes for a given amount of substance to become reduced by half as a consequence of decay.
Marie Curie had a lead sealed radioactive laboratory, notebooks, cookbooks and furniture contaminated by radium are a testament of her contributions in the field.
She was the only person to win the Nobel Prize in both physics and chemistry.
She was born in Warsaw, in what was then the Kingdom of Poland, part of the Russian Empire. She studied at Warsaw's clandestine Flying University and began her practical scientific training in Warsaw. In 1891.
Flying University was Polish patriotic institution of higher learning that admitted women students.
Marie won the 1911 Nobel Prize in Chemistry for her discovery of the elements polonium and radium, using techniques she invented for isolating radioactive isotopes.
On both the paternal and maternal sides, the family had lost their property and fortunes through patriotic involvements in Polish national uprisings aimed at restoring Poland's independence (the most recent had been the January Uprising of 1863–65).
In 1896, Henri Becquerel discovered that uranium salts emitted rays that resembled X-rays in their penetrating power.[30] He demonstrated that this radiation, unlike phosphorescence, did not depend on an external source of energy but seemed to arise spontaneously from uranium itself. Influenced by these two important discoveries, Curie decided to look into uranium rays as a possible field of research for a thesis.
Using her husband's electrometer, she discovered that uranium rays caused the air around a sample to conduct electricity. Using this technique, her first result was the finding that the activity of the uranium compounds depended only on the quantity of uranium present. She hypothesized that the radiation was not the outcome of some interaction of molecules but must come from the atom itself. This hypothesis was an important step in disproving the assumption that atoms were indivisible.
ESPCI Paris is a prestigious grande école founded in 1882 by the city of Paris, France. It educates undergraduate and graduate students in physics.
The Curies did not have a dedicated laboratory; most of their research was carried out in a converted shed next to ESPCI. The shed, formerly a medical school dissecting room, was poorly ventilated and not even waterproof. They were unaware of the deleterious effects of radiation exposure attendant on their continued unprotected work with radioactive substances. ESPCI did not sponsor her research, but she would receive subsidies from metallurgical and mining companies and from various organizations and governments.
In July 1898, Curie and her husband published a joint paper announcing the existence of an element they named "polonium", in honour of her native Poland, which would for another twenty years remain partitioned among three empires (Russian, Austrian, and Prussian). On 26 December 1898, the Curies announced the existence of a second element, which they named "radium", from the Latin word for "ray". In the course of their research, they also coined the word "radioactivity".
Pierre and Marie Curie, c. 1903
To prove their discoveries beyond any doubt, the Curies sought to isolate polonium and radium in pure form.[Pitchblende is a complex mineral; the chemical separation of its constituents was an arduous task. The discovery of polonium had been relatively easy; chemically it resembles the element bismuth, and polonium was the only bismuth-like substance in the ore. Radium, however, was more elusive; it is closely related chemically to barium, and pitchblende contains both elements. By 1898 the Curies had obtained traces of radium, but appreciable quantities, uncontaminated with barium, were still beyond reach. The Curies undertook the arduous task of separating out radium salt by differential crystallization. From a tonne of pitchblende, one-tenth of a gram of radium chloride was separated in 1902. In 1910, she isolated pure radium metal. She never succeeded in isolating polonium, which has a half-life of only 138 days.
The Curie's worked a great deal on what became known as the field of Atomic Physics.
Lead to the discovery of Radioactive elements like
Uranium-235 and Plutonium 239 when bombarded with neutrons release an enormous amount of energy.
Just 1 Kilogram of Uranium-235 can produce almost 24 million Kilowatt hours of power [kWh] through nuclear fission.
Remember The kilowatt-hour is a unit of energy equal to one kilowatt of power sustained for one hour.
1 Kilogram of coal can only produce 8 kWh of power.
This is why nuclear scientists have said that nuclear energy can power entire cities for decades.
The power and potential of carefully handled radioactivity and nuclear energy is immense.
Radioactivity is said to come with risks.
Radiation as a whole isn't dangerous.
Light bouncing off a reflective surface like the microwaves bouncing off food or the signals received by cell phones are just some forms of radiation.
One form that is said to be harmful to all biological forms. . .this is ionizing or nuclear radiation.
Radioactive material during it's decay process is said to emit ionizing radiation.
It is said that when a living being is exposed is exposed to such high radiation, it doesn't make them radioactive, but is said to cause radioactive poisoning.
It is said this can destroy the structure of dna and harm living cells.
It is said a heavy or prolonged dose can prove lethal.
These rays are said to be carcinogenic.
Radioactivity is not always harmful.
Is it the amount that can make it poisonous?
We are told regulated doses of radiation can cure cancer.
Radioactive Iodine is used in radiation therapy to treat cancer and for imaging in the thyroid gland.
Radioactive technetium is used for the detection of heart, bone and other organ defects.
Radioactive C-14 Carbon is used in carbon dating.
In some countries produce is lightly radioactive radiated to kill off the germs on fruits and vegetables.
A tiny amount of Americium-231 is used in smoke alarms.
Back to the Cesium Clock
Inside a cesium atomic clock, cesium atoms are funneled down a tube where they pass through radio waves . If this frequency is just right 9,192,631,770 cycles per second then the cesium atoms "resonate" and change their energy state.
A detector at the end of the tube keeps track of the number of cesium atoms reaching it that have changed their energy states. The more finely tuned the radio wave frequency is to 9,192,631,770 cycles per second, the more cesium atoms reach the detector.
The detector feeds information back into the radio wave generator. It synchronizes the frequency of the radio waves with the peak number of cesium atoms striking it. Other electronics in the atomic clock count this frequency. As with a single swing of the pendulum, a second is ticked off when the frequency count is met.
The first quality atomic clocks made in the 1950s were based on cesium, and such clocks honed to greater precisions over the decades remain the basis used to keep official time throughout the world.
In the United States, the top clocks are maintained by the National Institutes of Standards and Technology (NIST) in Boulder, Colo., and the United States Naval Observatory (USNO) in Washington, D.C.
The NIST-F1 cesium atomic clock can produce a frequency so precise that its time error per day is about 0.03 nanoseconds, which means that the clock would lose one second in 100 million years.
Super-accurate timekeeping is integral to many elements of modern life, such as high-speed electronic communications, electrical grids and the Global Positioning System (GPS) and of course knowing when your favorite television show comes on.
Caesium (IUPAC spelling[6]) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of 28.5 °C (83.3 °F), which makes it one of only five elemental metals that are liquid at or near room temperature.
Caesium has physical and chemical properties similar to those of rubidium and potassium. The most reactive of all metals, it is pyrophoric and reacts with water even at −116 °C (−177 °F). It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors.
The first small-scale applications for caesium were as a "getter" in vacuum tubes and in photoelectric cells.
Since the 1990s, the largest application of the element has been as caesium formate for drilling fluids, but it has a range of applications in the production of electricity, in electronics, and in chemistry.
The radioactive isotope caesium-137 has a half-life of about 30 years and is used in medical applications, industrial gauges, and hydrology. Nonradioactive caesium compounds are only mildly toxic, but the pure metal's tendency to react explosively with water means that caesium is considered a hazardous material, and the radioisotopes present a significant health and ecological hazard in the environment.
See more here,
https://www.livescience.com/32660-how-does-an-atomic-clock-work.html
Caesium metal is highly reactive and very pyrophoric. It ignites spontaneously in air, and reacts explosively with water even at low temperatures, more so than the other alkali metals (first group of the periodic table). It reacts with ice at temperatures as low as −116 °C (−177 °F). Because of this high reactivity, caesium metal is classified as a hazardous material. It is stored and shipped in dry, saturated hydrocarbons such as mineral oil. It can be handled only under inert gas, such as argon. However, a caesium-water explosion is often less powerful than a sodium-water explosion with a similar amount of sodium. This is because caesium explodes instantly upon contact with water, leaving little time for hydrogen to accumulate. Caesium can be stored in vacuum-sealed borosilicate glass ampoules. In quantities of more than about 100 grams (3.5 oz), caesium is shipped in hermetically sealed, stainless steel containers.
This song Sure says a LOT.
Never heard it before, but sure resonates with what we as #Messengers have been trying to tell #TheOthers for a long time now.
Michael Jackson/ the Jacksons..Can you feel it... with Lyrics
More sources,
https://opentextbc.ca/universityphysicsv3openstax/chapter/electron-spin/
https://arxiv.org/abs/2112.01086?context=quant-ph
https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.125.183001
Floquet Engineering
https://arxiv.org/abs/2203.03387
The power and versatility of this framework is demonstrated here by utilizing the independent-electron tight-binding description of the π electronic system of graphene. We show several prototype examples focusing on the region around the K (Dirac) point of the Brillouin zone: creation of a gap with opposing flat valence and conduction bands, creation of a gap with opposing concave symmetric valence and conduction bands -- which would correspond to a material with an effective negative electron-hole mass --, or closure of the gap when departing from a modified graphene model with a non-zero field-free gap. We employ time periodic drives with several frequency components and polarizations, in contrast to the usual monochromatic fields, and use control theory to find the amplitudes of each component that optimize the shape of the bands as desired. In addition, we use quantum control methods to find realistic switch-on pulses that bring the material into the predefined stationary Floquet band structure, i.e. into a state in which the desired Floquet modes of the target bands are fully occupied, so that they should remain stroboscopically stationary, with long lifetimes, when the weak periodic drives are started. Finally, we note that although we have focused on solid state materials, the technique that we propose could be equally used for the Floquet engineering of ultracold atoms in optical lattices, and to other non-equilibrium dynamical and correlated systems.
https://arxiv.org/abs/1909.02008
We review methods for using time-periodic fields (e.g., laser or microwave fields) to induce non-equilibrium topological phenomena in quantum many-body systems. We discuss how such fields can be used to change the topological properties of the single particle spectrum, and key experimental demonstrations in solid state, cold atomic, and photonic systems. The single particle Floquet band structure provides a stage on which the system's dynamics play out; the crucial question is then how to obtain robust topological behaviour in the many-particle setting.
https://www.science.org/doi/10.1126/science.1239834 This article from 2013
The replicas were seen at expected energy shifts, along with the gaps predicted to occur at the new energy-level crossings caused by the appearance of the replicas. Because Bi2Se3 is a topological insulator, the breaking of the time-reversal symmetry caused by circularly polarized light resulted in the appearance of an energy gap at the Dirac point, indicating an interesting route toward manipulating electronic states in such materials.
Observation of Time-Crystalline Eigenstate Order on a Quantum Processor
https://arxiv.org/abs/2107.13571
Observation of discrete time-crystalline order in a disordered dipolar many-body system
https://www.nature.com/articles/nature21426
More recently, advances in the controlled manipulation of isolated many-body systems have enabled detailed studies of non-equilibrium phases in strongly interacting quantum matter3,4,5,6; for example, the interplay between periodic driving, disorder and strong interactions has been predicted to result in exotic ‘time-crystalline’ phases7, in which a system exhibits temporal correlations at integer multiples of the fundamental driving period, breaking the discrete time-translational symmetry of the underlying drive.
Here we report the experimental observation of such discrete time-crystalline order in a driven, disordered ensemble of about one million dipolar spin impurities in diamond at room temperature13,14,15. We observe long-lived temporal correlations, experimentally identify the phase boundary and find that the temporal order is protected by strong interactions. This order is remarkably stable to perturbations, even in the presence of slow thermalization16,17. Our work opens the door to exploring dynamical phases of matter and controlling interacting, disordered many-body systems.
Observation of a discrete time crystal
An example is the breaking of spatial translational symmetry, which underlies the formation of crystals and the phase transition from liquid to solid. Using the analogy of crystals in space, the breaking of translational symmetry in time and the emergence of a ‘time crystal’ was recently proposed2,3, but was later shown to be forbidden in thermal equilibrium4,5,6. However, non-equilibrium Floquet systems, which are subject to a periodic drive, can exhibit persistent time correlations at an emergent subharmonic frequency.
This new phase of matter has been dubbed a ‘discrete time crystal’. Here we present the experimental observation of a discrete time crystal, in an interacting spin chain of trapped atomic ions.
e apply a periodic Hamiltonian to the system under many-body localization conditions, and observe a subharmonic temporal response that is robust to external perturbations. The observation of such a time crystal opens the door to the study of systems with long-range spatio-temporal correlations and novel phases of matter that emerge under intrinsically non-equilibrium conditions.
https://www.nature.com/articles/nature21413
From Science 6.11.2021
Prethermal time crystal
Characterizing and understanding different phases of matter in equilibrium is usually associated with the process of thermalization, where the system equilibrates. Recent efforts probing nonequilibrium systems have revealed that periodic driving of the system can suppress the natural tendency for equilibration yet still form new, nonequilibrium phases. Kyprianidis et al. used a quantum simulator composed of 25 trapped ion qubits and spins to observe such a nonequilibrium phase of matter: the disorder-free prethermal discrete time crystal. The flexibility and tunability of their quantum simulator provide a powerful platform with which to study the exotic phases of matter.
Extending the framework of statistical physics to the nonequilibrium setting has led to the discovery of previously unidentified phases of matter, often catalyzed by periodic driving. However, preventing the runaway heating that is associated with driving a strongly interacting quantum system remains a challenge in the investigation of these newly discovered phases. In this work, we utilize a trapped-ion quantum simulator to observe the signatures of a nonequilibrium driven phase without disorder—the prethermal discrete time crystal. Here, the heating problem is circumvented not by disorder-induced many-body localization, but rather by high-frequency driving, which leads to an expansive time window where nonequilibrium phases can emerge. Floquet prethermalization is thus presented as a general strategy for creating, stabilizing, and studying intrinsically out-of-equilibrium phases of matter.
https://www.science.org/doi/10.1126/science.abg8102
From SciPost Physics Core
Nonequilibrium steady states in the Floquet-Lindblad systems: van Vleck's high-frequency expansion approach
This theory is based on the high-frequency (HF) expansion with linear algebraic numerics and without numerically solving the time evolution.
In particular, for the isotropic Heisenberg chain, we propose the dissipation-assisted terahertz (THz) inverse Faraday effect in quantum magnets. Our theoretical framework applies to various time-periodic Lindblad equations that are currently under active research.
See more here,
https://www.scipost.org/SciPostPhysCore.4.4.033?acad_field_slug=astronomy
In Nature Physics
Floquet prethermalization in dipolar spin chains
Periodically driven Floquet quantum systems could provide a promising platform to investigate novel physics out of equilibrium1, but the drive generically heats the system to a featureless infinite-temperature state2,3,4. Fortunately, for high driving frequency, the heat absorption rate has been theoretically predicted to be exponentially small, giving rise to a long-lived prethermal regime that exhibits all the intriguing properties of Floquet systems
https://www.nature.com/articles/s41567-020-01120-z
So let's end with some info from the title. . .Ingersoll made a Sweepster watch and as shown in the following vid. . .the chronograph hand sweeps around back to the number it is calibrated to.
"The Second Hand UNWINDS?"
A lyric in the Cyndi Lauper song. . .
Time After Time (Cyndi Lauper) - Sam Tsui & Casey Breves | Sam Tsui
Quietdrive - Time After Time
Matchbox Twenty - Time After Time (Live)
Radium atomic number 88
Radium Salts used in the hands of the Radiolite pocket watch
Did not need an external source to glow as the radium reacted with the nitrogen in the air for the "Spark."
8.8.2022 Lions Gate in the skies!
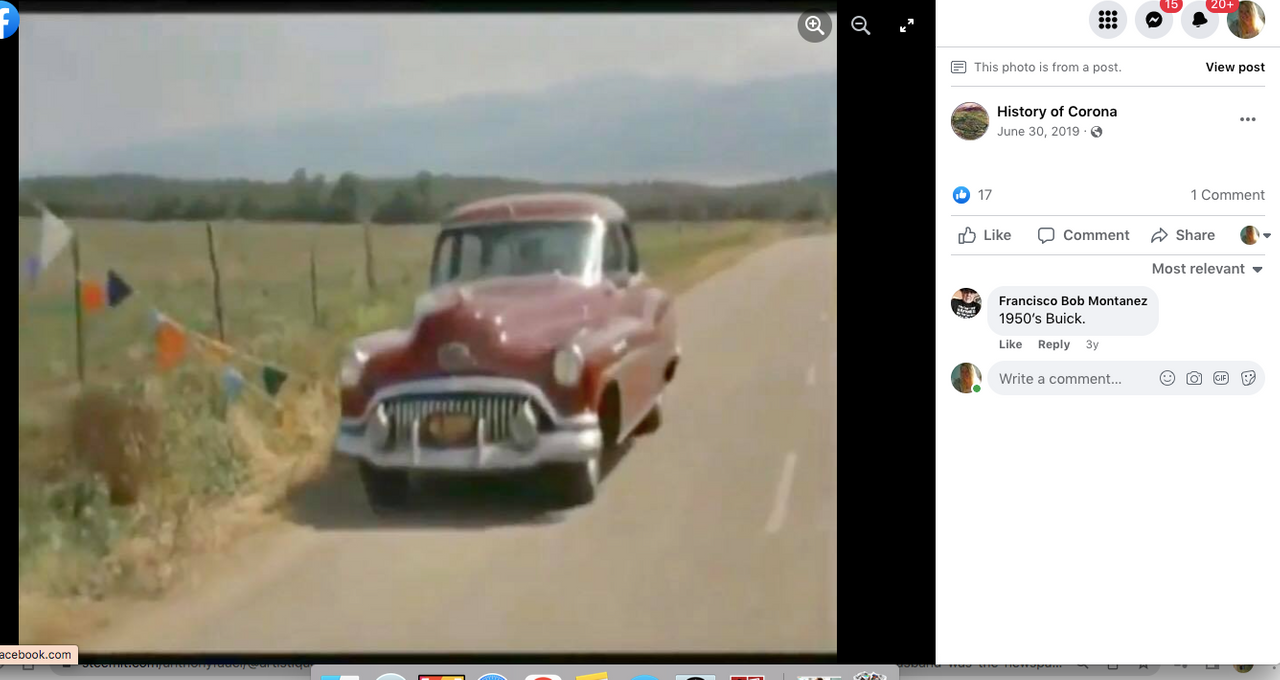
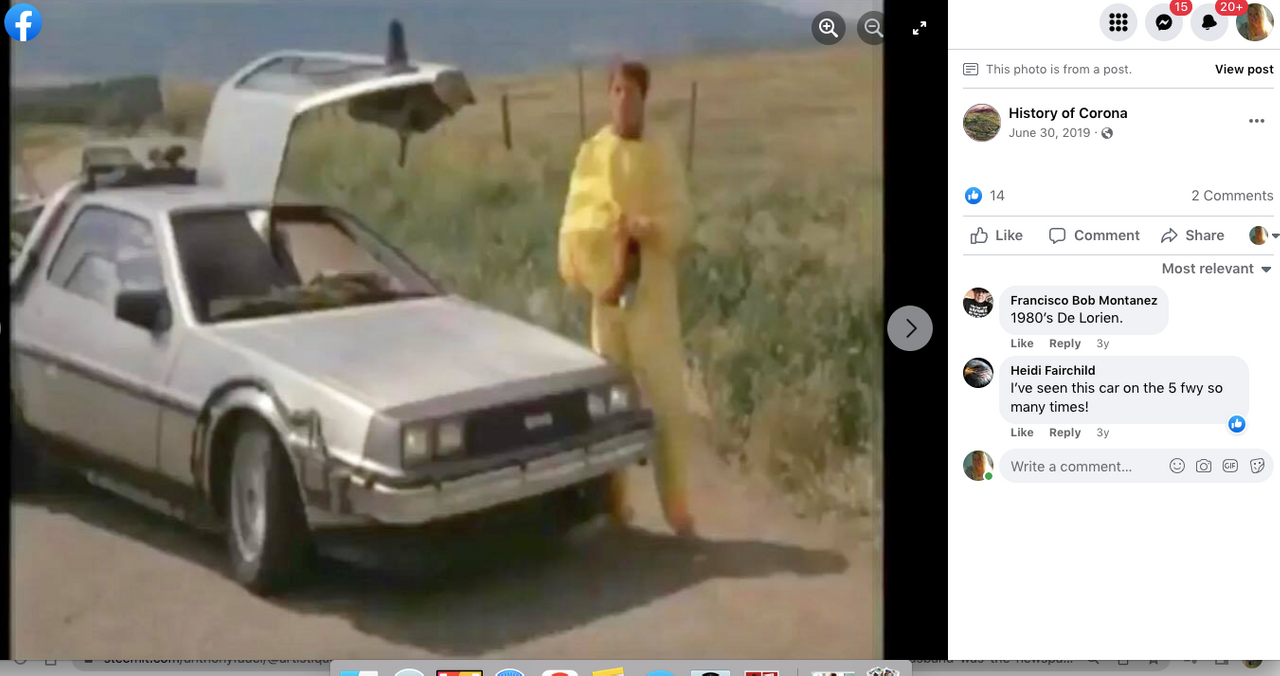
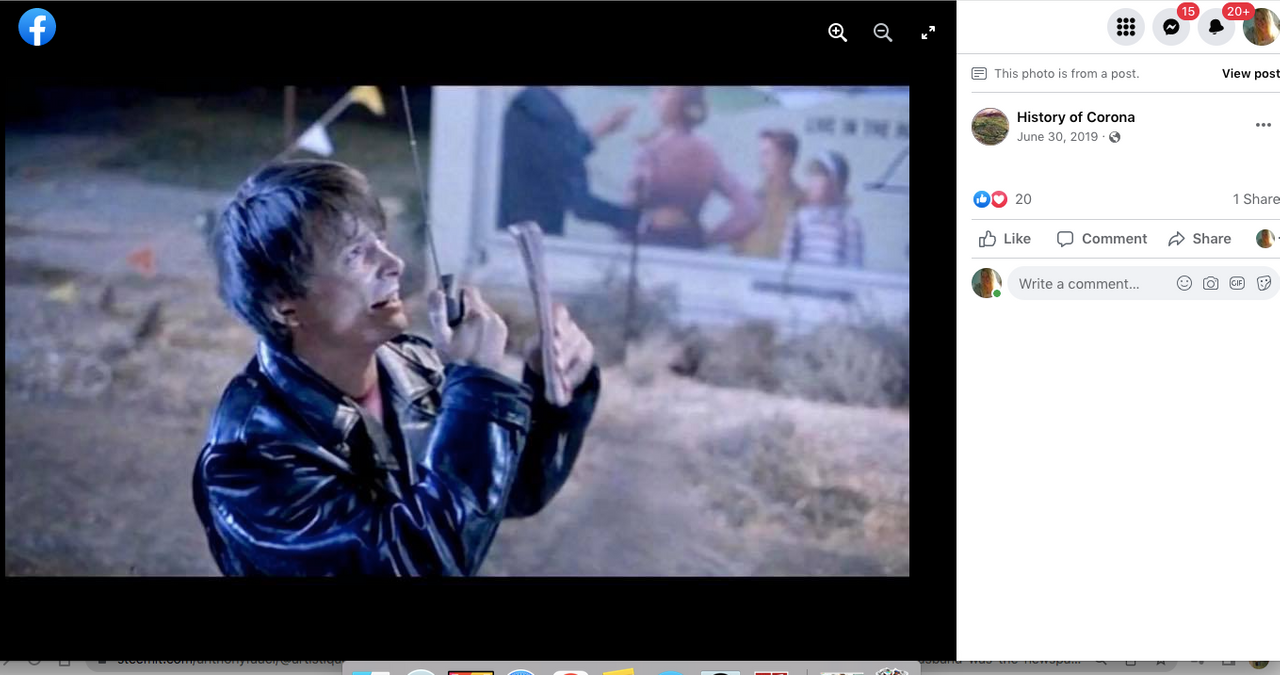
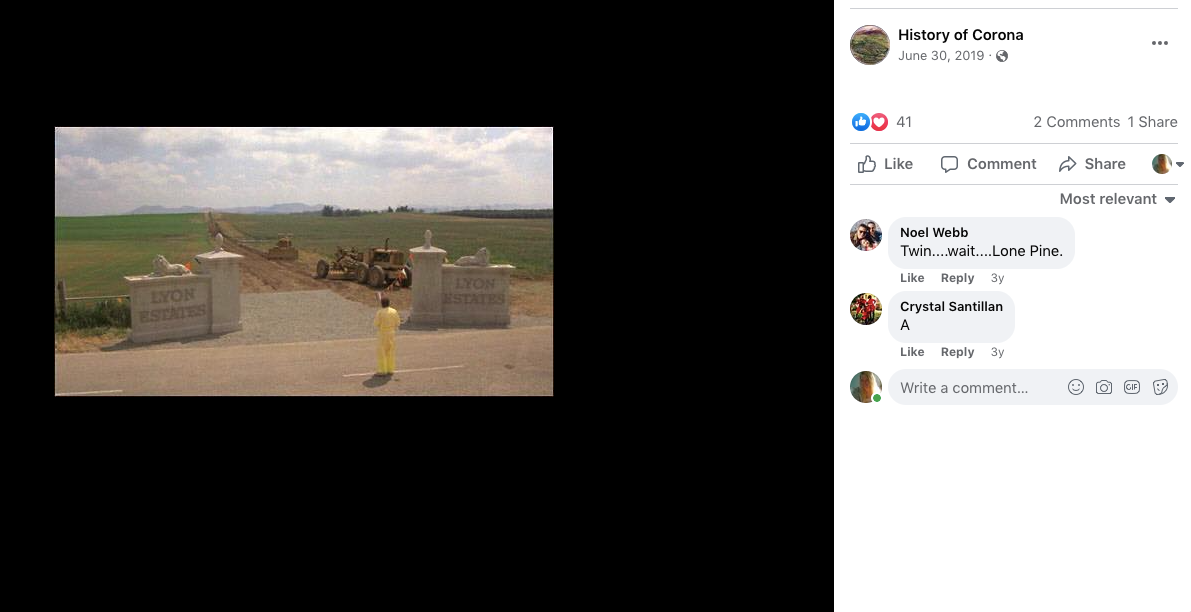
Check out back to the future and the name of the estates where Marty hides his Delorean behind the sign of the future Site being built. . .

Back to the Future | PART III | 1985 [Return to the Lyon Estates]

TDW 1205 - BTTF Lyon Estates 1955 with Delorean !

Back to the Future III
Check it out. . .#Corona Connect? You decide!
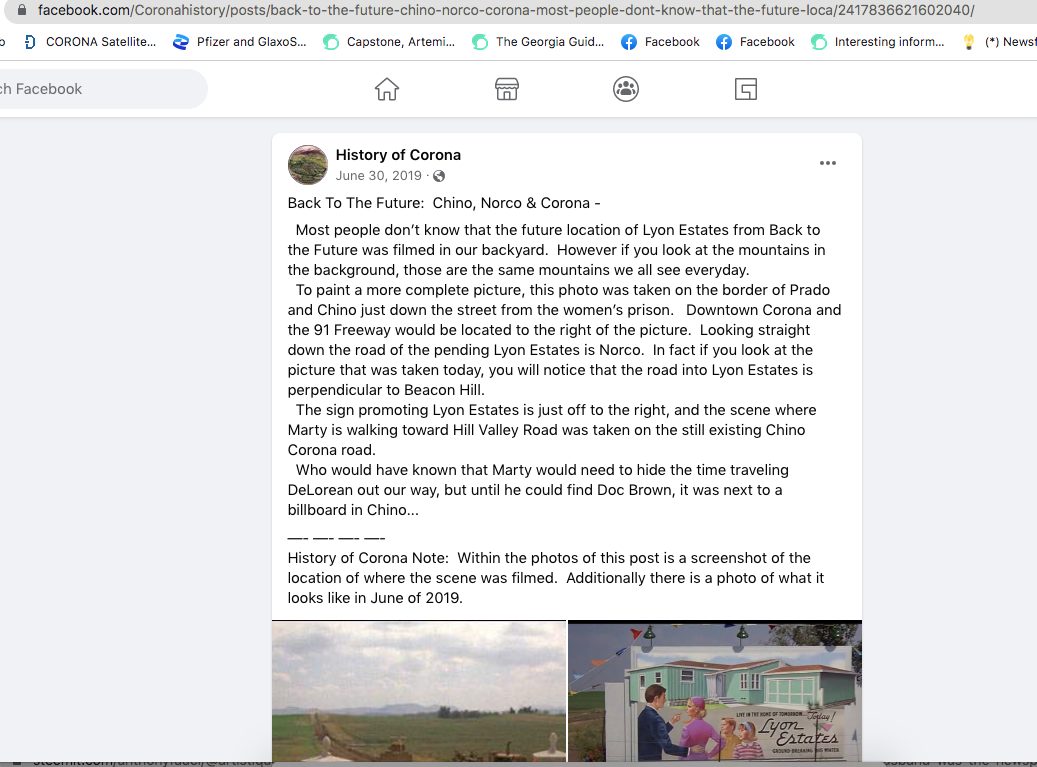
Back To The Future: Chino, Norco & Corona -
Most people don’t know that the future location of Lyon Estates from Back to the Future was filmed in our backyard. However if you look at the mountains in the background, those are the same mountains we all see everyday.
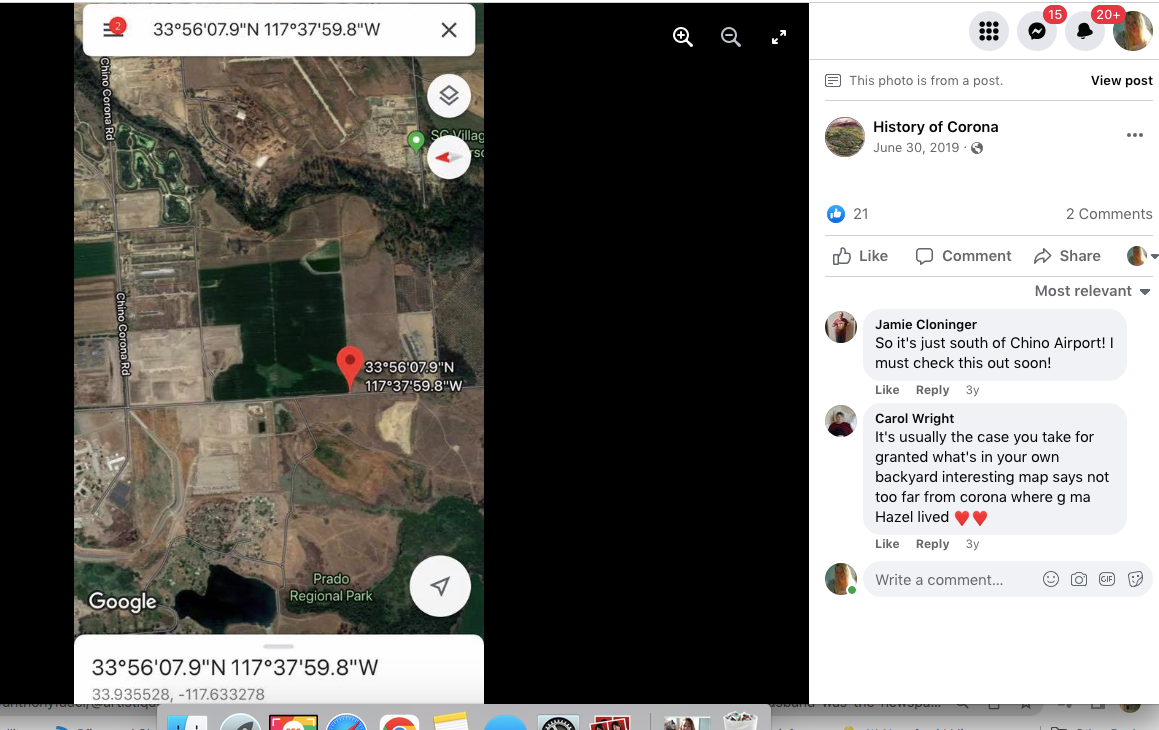
To paint a more complete picture, this photo was taken on the border of Prado and Chino just down the street from the women’s prison. Downtown Corona and the 91 Freeway would be located to the right of the picture. Looking straight down the road of the pending Lyon Estates is Norco. In fact if you look at the picture that was taken today, you will notice that the road into Lyon Estates is perpendicular to Beacon Hill.
The sign promoting Lyon Estates is just off to the right, and the scene where Marty is walking toward Hill Valley Road was taken on the still existing Chino Corona road.
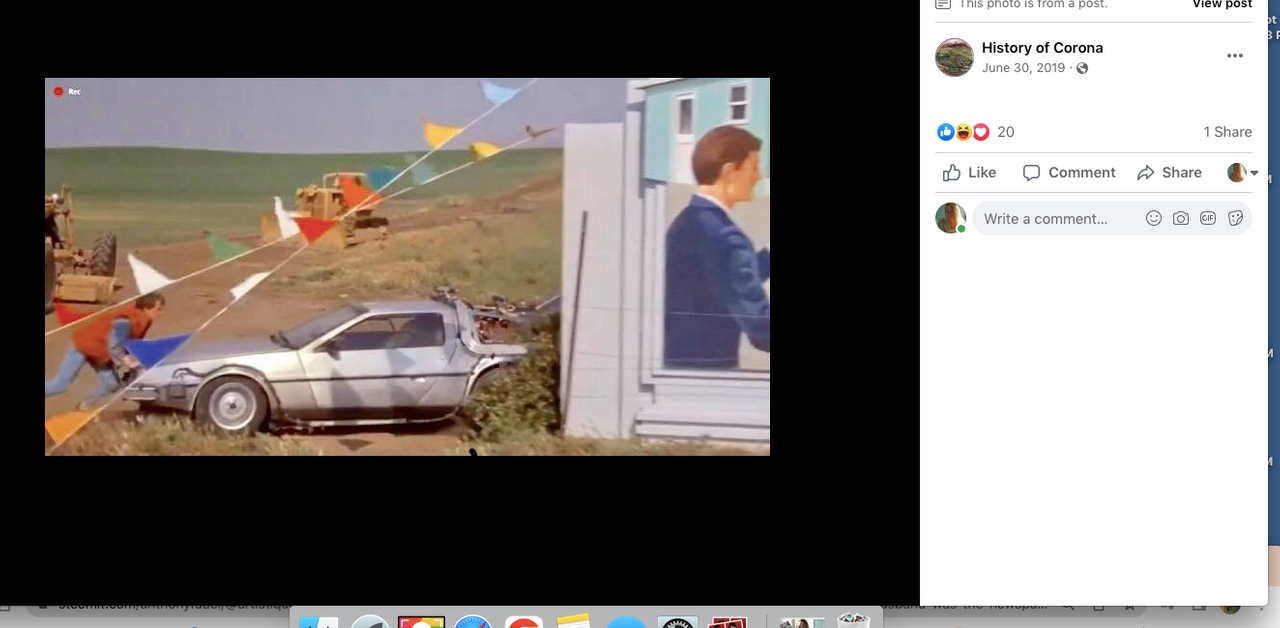
Who would have known that Marty would need to hide the time traveling DeLorean out our way, but until he could find Doc Brown, it was next to a billboard in Chino...
From this post. . .
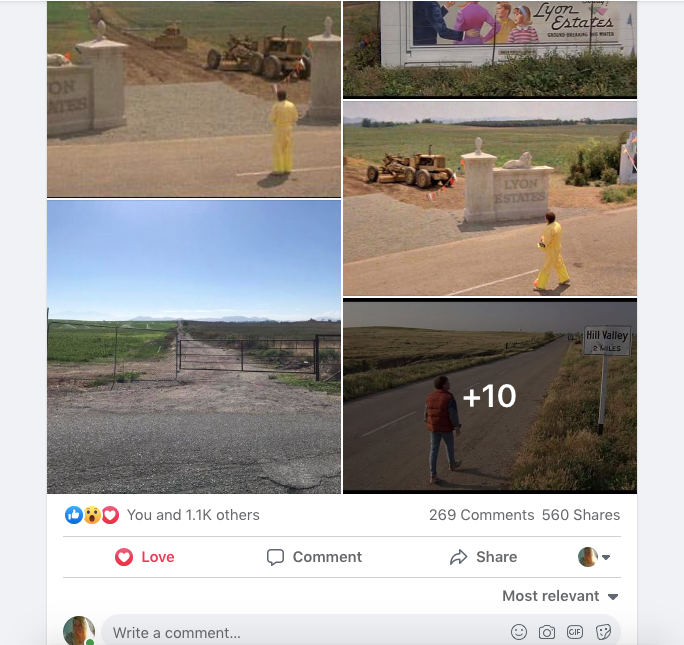
History of Corona Note: Within the photos of this post is a screenshot of the location of where the scene was filmed. Additionally there is a photo of what it looks like in June of 2019.
I am archiving this post here with evidence,




Time After Time - Cyndi Lauper | Caleb Grimm Acoustic Cover
I Will Remember You - Sarah Mclachlan | Caleb Grimm Acoustic Cover
See the above thread here,