Introduction: Keeping it together
Have you ever wondered what makes you an individual? There might be several reasons, but some of it has to do with the fact that you don't just bleed into other organisms and people around you. If you did that, hugs would be awkward: you'd walk away from a good warm hug carrying somebody else's head, and they'd have your arms...it'd be a mess. Something has to keep your insides inside, and your outsides outside.
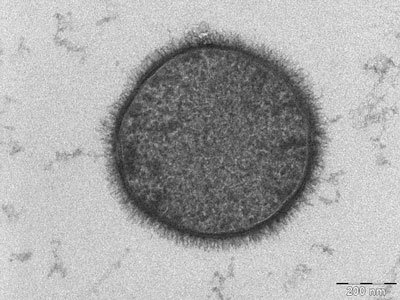
TEM micrograph of a B. subtilis cell. No bleeding out: keeping it all in the family!
Source: Wikimedia Commons contributors
Is it your skin? Well, if you look closer, your skin is just an organ in a system of organs called the integumentary organ system [1: intro]. And the skin is further comprised of tissues — 7 of them, in fact [ibid.] And then there's cells...
Actually, that's where I want to stop. The cell. It's the smallest thing that can be called living, and the smallest living thing that can individuate itself: separate its insides from its outsides. We — macrohumans — are just collections of cells, so naturally we inherit their properties.
So maybe if we can understand how cells keep it — their inner goo — together, maybe we can learn how we keep it together.

Let's find out how we managed to avoid this poor man's fate.
Source: giphy
A chemical detour
I profusely apologize. I really do. But think of it this way: at least it's not math! And, it's a detour, not a main destination. It'll help us better appreciate our final destination (don't worry, no one's getting killed), which is biology. So yay!
6 out of 118
Currently, there's a hundred and eighteen (that's 118) elements in the periodic table. Biologists mainly care about 6 (that's six) of them [2: chapter 2], and only 4 of them — carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) — make up more than 96% of an organism's weight [3]. So already you're getting a sweet deal. Here they are, the magnificent 6:

Is it me, or does the N shift a pixel to the right?
Source: Wikimedia Commons contributors, modified.
Shrewd chemists will notice I've replaced the atomic number with the number of covalent bonds the atoms can form. That's because that's one of the numbers biologists most care about [2: chapter 2, section 3].
Now what are covalent bonds, you ask. I won't go into the chemical details, but covalent bonds are basically what hold atoms together strong enough to form larger units called molecules, and those are essential for life [ibid.] Water, after all, is a collection of water molecules, and we are 70% water [2: chapter 2, section 2]. Remember the dude up there in the gif?
Atoms can form many bonds, not just covalent ones. They have a kind of chemical romance thing going on. They can form ionic bonds, and hydrogen bonds [2: chapter 2, section 1], and I won't go into all that. They're just bonds, keeping atoms together. But if you want to remember the number of covalent bonds these 6 atoms can form, here's some useful mnemonic devices.
You can skip this section
(See 3 in references for the number of covalent bonds these atoms use.)
Here's the mnemonic devices I use. Hydrogen (H) and Oxygen (O) are easy to remember: together they form one of the most important molecules for life, H2O. 2 Hydrogens covalently bond with 1 Oxygen, because Oxygen can form 2 covalent bonds, and Hydrogen can only form 1.
Nitrogen is a shifty one. Another word for shifty is coNNiving. Let's just straight out memorize this.
Carbon (C) can form 4 covalent bonds, and if you can remember the explosive C4, you're set.
Phosphorus (P) can form 5 covalent bonds. The number 5 in Greek is pente. That's where pentose (5 carbon sugar) gets its name. Or pentecostal. Or pentachord. There's probably simpler words out there that begin with penta-, but I'm not here to make your life easy. P = pente = 5, is what I'm trying to say.
Sulfur (S) can form six covalent bonds. Now what's a word that begins with the letter s and denotes the number six? Hmm. Let's go with Sistine Chapel. Everyone knows it gets its name from Pope Sixtus IV. It's obvious how the number 6 relates to that name: if you add II to IV, you get VI. So Sulfur can form VI covalent bonds. Best. Mnemonic. Device. Ever. And I'll be damned if you can find a better one.
Greediness
(For all discussions from hereon about polarity and electronegativity, see 3 in references.)
You know how some kids want everything for themselves, whereas other kids are more likely to share their toys? Turns out, that's true of atoms too. Some atoms are more electronegative — excuse me, I meant greedy, I used the proper term there for a second, let's call it an academic slip — greedier than others, so they form polar bonds. Remember H2O? That's a polar covalent bond, because the Oxygen is more electronegative — arg! damn you, books! — because the Oxygen is greedier than the hydrogens.
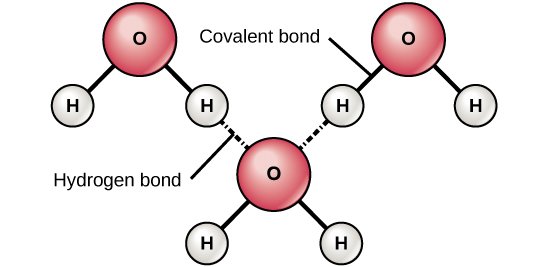
Source: OpenStax, Concepts of Biology
Bonds that share equally are called nonpolar bonds. The bond between a Carbon and another Carbon for instance (represented like so: C—C) is a nonpolar bond. Why? Because logic: they're the same exact atom, so they have the same exact properties, so they can't be greedier than their partner.
But also, they're just not very electronegative even when they're not partnered up with their equals. That's just how the Carbon rolls.
Rule of thumb: when a molecule has Oxygens or Nitrogens, it's polar. When it has any of the rest of the magnificent 6, it's nonpolar.
Polar and nonpolar don't mix
Now what happens when you're a nonpolar bond, and you find yourself in the midst of a bunch of water molecules wanting to interact with one another, the Oxygen orienting itself so it can form hydrogen bonds with Hydrogens from other water molecules, like the picture above?
Well, let's think of it macrohumanly. What would happen if you were in a crowd where everyone wanted to hug each other, but no one wanted to hug you? Exactly: you'd be slowly pushed to the outside of the group.
Hey, did I just explain why water and oil don't mix? I guess I did!

I'm so Greek, if that were olive oil, I'd squeeze some fresh lemon juice in it, and drink it.
Source: Wikimedia Commons contributors
Another name for polar and nonpolar
Polar molecules like to interact with water. They are welcomed, because they can hug. So they're called hydrophilic (hydro meaning water in Greek, philic meaning loving). Salt can dissolve in water, because it's hydrophilic.
Nonpolar molecules don't like to interact with water. So they're called hydrophobic (phobic meaning fearing).
Fence-sitters
Some molecules just don't wanna commit. They're rebels like that. Part of them is philic, part of them is phobic. We call these amphipathic. I'd call them bipolar, but someone got there before me and planted his moon flag.
We now got all the chemistry we need to proceed with the biology! Detour over! Yay!
By the way, in case you're wondering why all this insistence on water, consider this quote from the Molecular Biology of the Cell: "Life on Earth began in the ocean, and the conditions in that primeval environment put a permanent stamp on the chemistry of living things. Life therefore hinges on the properties of water." [3]
Now we know!
Amphipathic
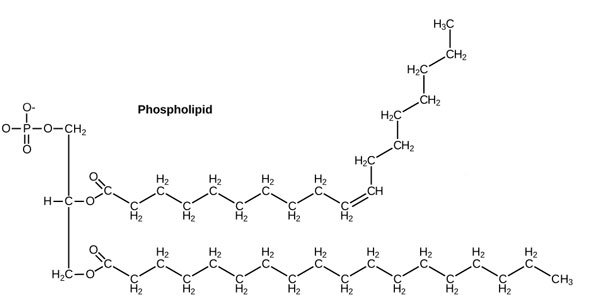
Source: OpenStax, Concepts of Biology, modified.
Now you know enough chemistry to know that the right part of this picture, with all those Carbons and Hydrogens, will be very no-thank-you toward water, whereas the left part of this picture, with all those Oxygens, will be very-happy-indeed-thank-you-very-much to interact with water.
Let's simplify that picture a little bit cos it looks scary. Let's make the philic part a happy smiley face (officially called a phosphate head), and the phobic part some kind of party-pooping black running-away line (officially called a tail) [4].
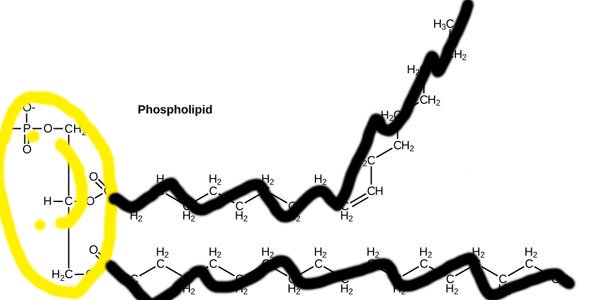
There, that looks much less scary and easier to comprehend. Or does it?
Source: OpenStax, Concepts of Biology, modified.
So what's gonna happen? Well, remember that scene in the movie Stuck on You where conjoined twin and womanizer Greg Kinnear was having sex with a woman he just picked up while his more sensitive conjoined brother Matt Damon was sending a romantic text to a love interest? That's more or less what would happen. But let's go into some more detail, because this is the gist of the article.
How membranes form spontaneously
Now this is the part that gets my heart pumping. I'm serious. This is the stuff I'd share around campfires while others share their ghost stories. Hey, my story might make some people sleepy, but at least it's true!
When you throw in an amphipathic molecule that has both a hydrophilic (water-loving) part and a hydrophobic (water-fearing) part into water, there's gonna be a problem. There's three ways that this problem can be resolved. In other words, there's three arrangements that the molecules can adopt that will resolve the tension. Way number one:
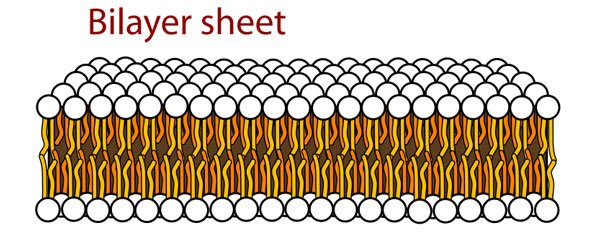
Source: Wikimedia Commons contributors, modified.
Hey, I know these guys! It's our good ol' friends the smileys with their tails! Arranged in a bilayer sheet configuration! That will keep their hydrophobic tails inside, leaving their hydrophilic heads to interact with the water surrounding them all they want! That's some smart thinking. Or, it would be, if it didn't happen spontaneously. Let's move on to way number two:

Source: Wikimedia Commons contributors, modified.
Hey, what do we got here! The amphipathic molecules have decided to take on a round shape. This is slowly starting to look like a regular cell. Hence the name I guess. Can't wait to see what comes next!
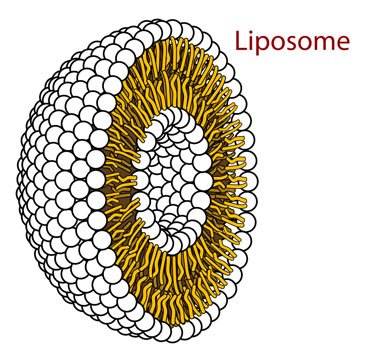
Source: Wikimedia Commons contributors, modified.
Dun dun dun! Enter the liposome. I can already imagine a nucleus housing a DNA molecule in that waterhole in the middle. Smart houses ain't got nothing on this. And what did we do to make it happen? Nothing: we just threw in some amphipathic molecules in water and shook them around. And I just added that last step for flourish: it's not really necessary.
So there you got it folks, the story of how membranes are made spontaneously. Obviously the cells that make you up are a little bit more involved, more like this:
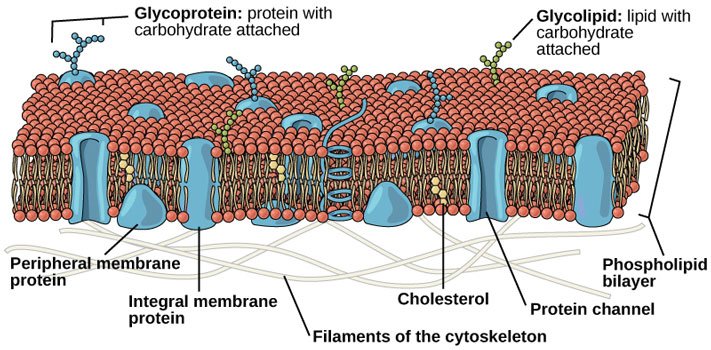
Source: OpenStax, Concepts of Biology, modified.
But you can still see our friends the smiley faces with tails playing a center role in there. To my eye, they're the most abundant thing in that picture.
Curtain close
In the previous "introduction to biology" post I examined vitalism, the idea that life was something beyond just chemistry and physics, and I showed the Nobel Prize experiment that blew that theory out of the yeast water. In this post, in keeping with that same mechanical approach to life, I explained how cell membranes form spontaneously simply because of the laws of chemistry. You don't need no angel dust!
I find it's easier to learn any subject, when we somehow relate it to our human inquiries and interests. Biology is not just about memorizing data. It's about understanding how we came to be here. It's about making sense of the world. And there's no better place to start — or end — than our cells.
And now we can go out there and hug people with not a worry in the world that we'll spill into each other!
References:
Wikipedia contributors, "Human skin," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Human_skin&oldid=808028388 (accessed November 4, 2017).
OpenStax, Concepts of Biology. OpenStax CNX. Jul 17, 2017 http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@9.25.
Molecular Biology of the Cell. 4th edition. https://www.ncbi.nlm.nih.gov/books/NBK26883/
Wikipedia contributors, "Lipid bilayer," Wikipedia, The Free Encyclopedia, https://en.wikipedia.org/w/index.php?title=Lipid_bilayer&oldid=800307900 (accessed November 4, 2017).
steemSTEM is the go-to place for science on Steemit. Check it out at @steemstem or browse the #steemSTEM tag or chat live at steemit.chat
