
Introduction
This informative series of posts will explore modern biology; the fundamental principles of how living systems work. This material will always be presented at the level of a first-year college biology course, without assuming any prior background in biology or science. It also presents material in a conceptual format. Emphasizing the importance of broad, unifying principles, facts and details in the context of developing an overarching framework. Finally, the series takes a historical approach wherever possible. Explaining how key experiments and observations led to our current state of knowledge and introducing many of the people responsible for creating the modern science of biology.
This post develops the argument that the most fundamental definition of information on the molecular level must involve a description for building proteins, which are the molecular “workhorses” of the cell.

We begin by describing common biochemical properties of the four major classes of biomolecules: lipids, carbohydrates, nucleic acids, and proteins. We then focus on proteins specifically, elaborating on the many functional roles proteins play in the life of the cell. Protiens act as catalysts, motors, signals, signal receivers, and structural supports, for example. Understanding these functions will emphasize the relationship between structure and function on the molecular level.
Biological information (DNA) serves as a “blueprint” in cells to build proteins.

Proteins are the workhorses of the cell; they play at least some role in almost every cellular process. Proteins form structural elements, acting as supports and girders to support cells. They form the cytoskeleton (cell skeleton). Proteins perform mechanical work by acting as small motors, changing cell shape, or transporting materials within cells. Proteins act as vehicles for other substances; for example, hemoglobin transports oxygen in the blood. Proteins act as signal receptors for molecular signals between cells, and they can act as the signals themselves. Proteins regulate cell functions by switching cellular processes on and off. Proteins also act as catalysts for chemical reactions; when they are engaged in this process, they are called enzymes. Enzymes make the chemical processes of life work in the environment of the cell. Proteins can perform so many different functions because there are many different kinds of proteins. In an E. coli cell, there may be 10,000 different proteins; in a typical human cell, 50,000.
The function of a particular protein is determined largely by its shape.
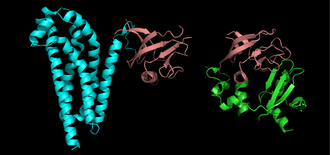
Proteins are strings of amino acids folded into three-dimensional shapes. The shape of a protein is often intuitively linked to its function; a structural protein will often be shaped like a girder, for example. Enzymes are shaped to fit other chemical compounds involved in a chemical reaction.
The chemical properties of amino acids provide a starting point for understanding protein shape and, therefore, function.
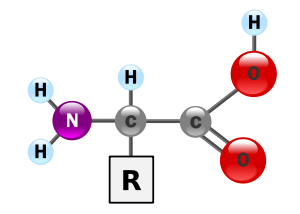
Each amino acid is a carbon atom surrounded by a carboxyl group, an amino group, and a hydrogen atom. Because carbon can form four chemical bonds, one bond is available. The fourth bond is attached to the side chain or R group, which can be a number of different chemical structures. The 20 different amino acids found in living systems are differentiated by their side chains. The physical and chemical characteristics of the side chain determine the unique characteristics of each amino acid and, therefore, contribute to the properties of a protein. Amino acids form strong peptide bonds between their carboxyl groups, forming polymer chains (polypeptides). Polypeptides have a “backbone,” formed by the repeating pattern of one nitrogen atom and two carbon atoms in each amino acid. One end of the polypeptide will have a free nitrogen (the Nterminus), while the other end will have a free carbon (the Cterminus). Each terminus is chemically different from the other, giving each polypeptide an inherent polarity. The length of a protein may be quite variable, from as few as 50 amino acids to as many as 5,000.
Polypeptides do not remain stretched out but, instead, bunch up into compact, globular, three-dimensional shapes.
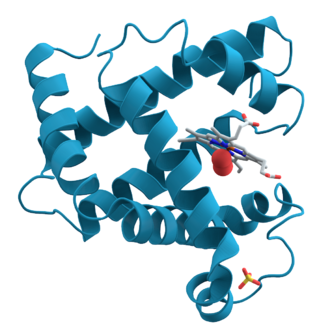
This complex shape determines protein function. The three-dimensional shape of a protein is determined by the linear sequence of its amino acids. The particular folding of a specific polypeptide is caused by the way in which the amino acid side chains interact with each other and with their (usually watery) environment. This interaction has both physical and chemical components.
Proteins have several structural levels, each more complex than the last.
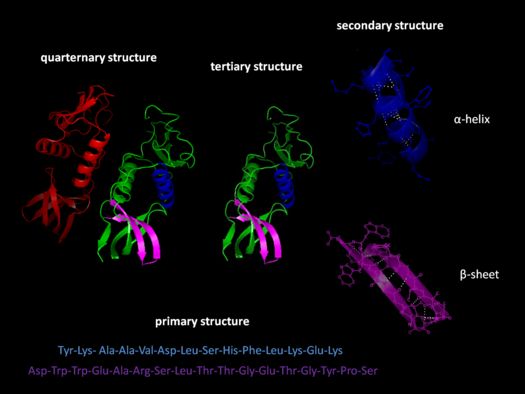
- A protein’s primary structure is simply the sequence of amino acids in the polypeptide chain. Early work in protein biochemistry focused on the primary structure.
- The secondary structure is the first level of folding that emerges as the carbon and nitrogen in the polypeptide backbone form very weak “hydrogen bonds” with each other.
- The tertiary structure begins to determine protein function. Because it is the result of interactions between the variable side chains of amino acids, the folding on this level can be complex.
- The quaternary structure occurs in proteins that comprise several polypeptides and results from the association of several different polypeptides with each other.
There are three kinds of interactions:

Some side chains are hydrophobic (“water hating”), while others are hydrophilic (“water loving”). Hydrophobic side chains twist to avoid water in the cell, ending up in the protein’s center, while hydrophilic side chains twist toward water and emerge on the protein’s surface. Water-based folding is a major determinant of protein shape.
Hydrogen bonds and other weak chemical bonds can form between side chains, further influencing the tertiary structure.
Certain kinds of amino acids can form strong covalent bonds with each other, changing and strengthening the tertiary structure.
The relationship between shape and function in proteins, coupled with the fact that a protein’s shape is determined largely by its primary structure, means that the amino acid sequence is the information that must be encoded, replicated, and transmitted to build proteins.
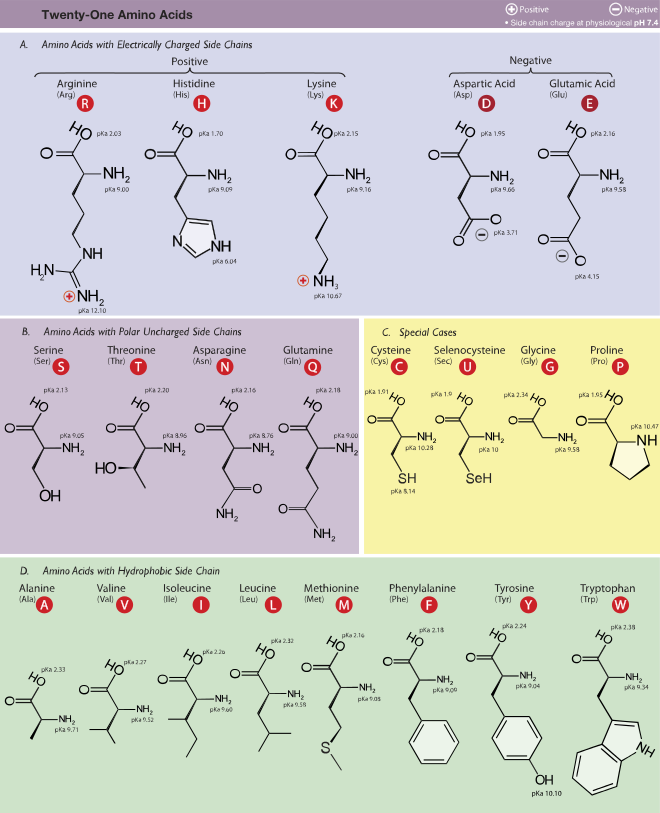
The function of a protein is determined by its three-dimensional shape, but the only aspect of a protein’s structure coded for by DNA is its linear sequence of amino acids. This linear storage of information is sufficient to encode 3-dimensional designs. There are many kinds of amino acids, but only 20 are found in naturally occurring proteins.
END PART 5
BIOLOGY THE STUDY OF LIFE:
PART 1 INTRODUCTION
PART 2 WHAT IS LIFE
PART 3 ORIGIN OF LIFE
PART 4 CELL TO ORGANISM
PART 5 PROTEINS
PART 6 CODE OF LIFE

 or
or  @pjheinz
@pjheinzImage Credits:
ALL IMAGES UNLESS NOTED - Pixabay
CC0 Public Domain
Free for commercial use
No attribution required
