Nature is truly magnificent but also unforgiving. Mankind struggled and almost did not make it. The invention of tools helped us to survive and also to strive. In this series I want to talk about some of the most sophisticated tools mankind has ever invented. Tools that help us to unravel the mysteries of nature and to understand our place in this universe.
Man is a tool-using animal. Without tools he is nothing, with tools he is all.
- Thomas Carlyle
Because I refer to it a lot in my Lab Chronicles Posts Day One and Day Two, I feel like it is time to explain how attenuated total internal reflection spectroscopy works. As always, I will try to keep explanations simple because I want to reach as many people as I can .

What is ATR Good For?
ATR can be used to identify what a surface is composed of. In my current research, I am attaching a layer of organic compounds to a gold surface. With ATR I can verify that the organic compounds have attached to the gold surface.
ATR provides you with a graph that shows you absorbance as a function of wavenumber. Now this sounds complicated, but is relatively easy to explain. Light is composed of many different wavelengths. We can perceive these different wavelengths (different colors are radiation at different wavelengths). However, there are many different wavelengths that we cannot perceive, like x-ray, infrared, ultraviolet and microwaves. For ATR, we are using wavelengths in the Infrared regions. To get the wavenumber from wavelength, you simply divide the number 1 by the wavelength.
Absorbance is also pretty easy to understand. Think of a mirror. You shine light on it and it gets reflected. In crude term, there no absorbance happening. Now think about a black surface. If you shine a light on that, you will get only very little reflection. You will also observe that the surface may heat up. This is due to absorbance.
Infrared absorbance is a little more complex than just regular light. Infrared radiation is absorbed differently by different chemical bonds. In short, ATR measures the absorbance of the surface at different wavenumbers. The wavenumber that is being absorbed is characteristic of a chemical bond. From the absorbance we can then determine which chemical bonds are present, and thus what is attached to our surface. It is a very useful technique!
Source
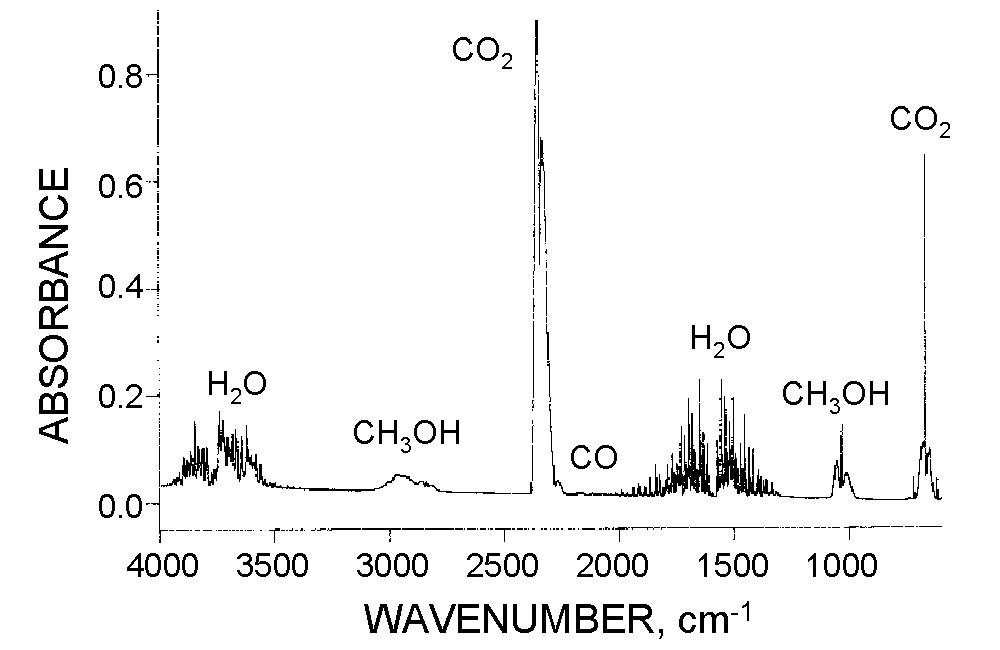
Here is a typical ATR spectrum showing absorbances at certain wavenumbers and the corresponding chemical bond associated with it.
ATR basically gives you the same information as the less sophisticated external reflection infrared spectroscopy, however, you get higher intensities in your data, which makes it easier to identify peaks.
How Does ATR Measure Absorbance at Different Wavenumbers
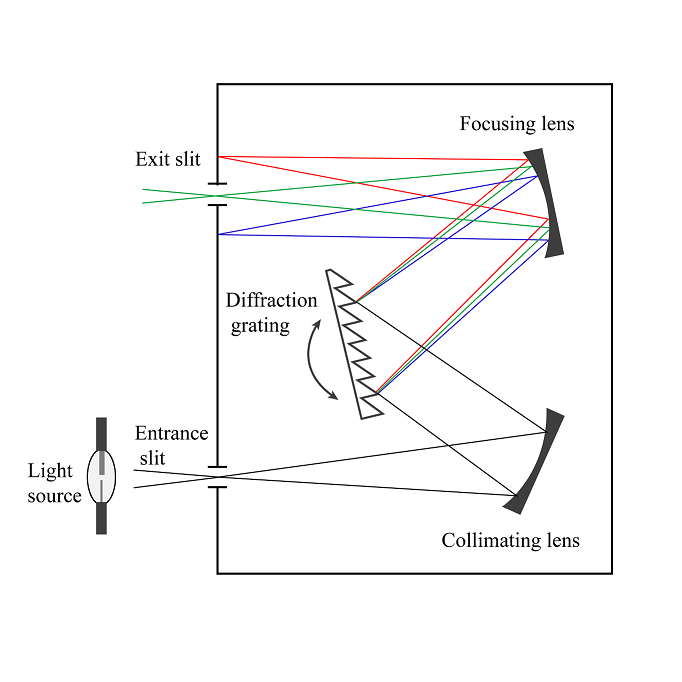
There is a source of radiation. Depending on the experiment you are performing, there are different sources. After the source, you usually have a wavlength selector (most of the time it is a monochromator). Wavelength selection is based on a grating. The grating, in very general terms, works like a prism. When you shine white light at a prism, the light gets broken into radiation of different wavelength (thus we see different colors). The grating does the same, it splits initial radiation into individual wavelengths (selection can be as precise as a fraction of a nanometer). Through adjustment of the grating alignment, we can focus each wavelength beam onto an exit slit. Here is a sketch of a monochromator.
Source
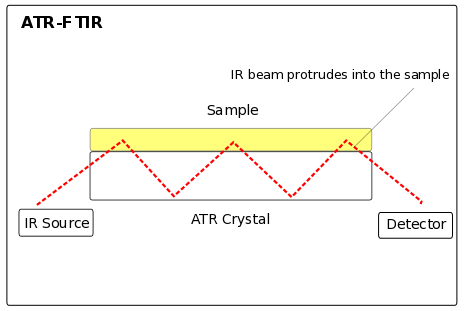
The selected wavelength is then send through a tube that functions like a fiber optic. Wavelength enters at a critical angle, is reflected at one side of the tube and then basically travels through the tube bouncing back and forth. The sample is places onto a Germanium crystal, which allows IR radiation to pass onto the sample. I took this picture in my laboratory.

At the sample the radiation interacts with the sample. I honestly do not know the full mechanism, so I will not go in much detail. The sample needs to be fixed onto a reflective surface, so that when the IR radiation reaches the sample and leaves the tube through the Germanium crystal, it gets reflected back into the tube so that it can be directed onto a detector. Here is an illustration of the process:
Source
Thank you very much for reading! I hope this helped you to understand this technique. If you enjoy reading science contents, please check out my blog. I have a series about Genius and Madness where I talk about scientists who had a mad side. Another series I have is about my daily lab work.
I want to provide quality content, so I appreciate feedback!
Stay curious.
Cheers @lesshorrible!

