As scientists and researchers seek to improve the quality and health of human life, we are often presented with scientific breakthroughs in the field of diseases prevention, and this time, via a process involving chemical modifications to the genes via base editor system.
In fact, a fresh new experiment conducted by Dr Puping Liang, et al, of Sun Yat-Sen University, Guangzhou - China on September 2017, allowed the correction of the faulty HBB gene causing β-Thalassemia, utilizing the base editor system.
Brief History
Scientific research has been conducted for years now towards the concept of editing faulty and disease causing genes at early stages of human development.
Several techniques had been in the works, the most famous of which is the CRISPR-Cas9.
Base editing, a process initiated by Professor David Liu of Harvard University few years back, and as best described by Alexis Komor, Post-doctoral fellow at Harvard University
is a new genome editing technology that enables the direct, irreversible conversion of a specific DNA base into another at a targeted genomic locus. Importantly, this can be achieved without requiring double-stranded DNA breaks (DSB).
While CRISPR-Cas9 converts base pairs of DNAs (quick reminder, DNA is a series of base pairs, or nucleotides: adenine (A), thymine (T), guanine (G) and cytosine (C) ), such as converting a C:G base pair to a T:A , the advantage base editing brings to the table is the capability to edit a single one of DNA’s bases, in this particular case converting A>G (in the HBB-28 gene).
Why β-Thalassemia?
β-Thalassemia is a life threatening genetic disease, caused by a mutation of the HBB gene.
This genetic disease is a global health concern, especially in the regions of North Africa, Middle East, India, Central and Southeast Asia.
As per the authors of the study:
Patients with β-thalassemia major usually die before age 5.
Hence the high magnitude of risk involved with the disease. Also per the authors:
Correcting this mutation in human embryos may prevent the disease being passed onto future generations and cure anemia
The Details
The base editor system used in performing this process is
a RNA-protein complex, adapted from the CRISPR/Cas9 system and cytidine deaminase
With the identified gene is HBB-28 and the correction needed is A>G as the single DNA base.
The subjects of the study were lab developed embryos infused with β-thalassemia affected cells.
Two different editors were studied before performing the work, and editor entitled BE3 was the one chosen for the process.
Image 1 below shows the first attempt at correction A>G at the HBB-28 gene applied onto human cell lines. This provided an outcome of respectively 16.3% and 26.0% G>A conversions for gRNA-1 and gRNA-2
these results clearly indicate the feasibility of repairing HBB −28 (A>G) in human cells in situ by base editing
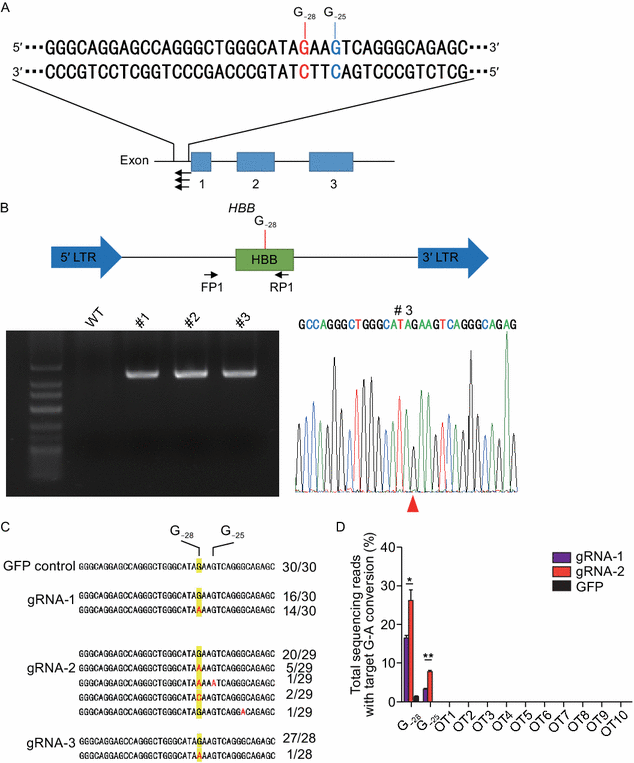
Image 2 below shows the second experiment applied on skin fibroblast cells of a patient, for the similar correction A>G at the HBB-28
under this experiment 17.8% cells was repaired precisely, demonstrating the feasibility of repairing HBB −28 (A>G) mutation in situ
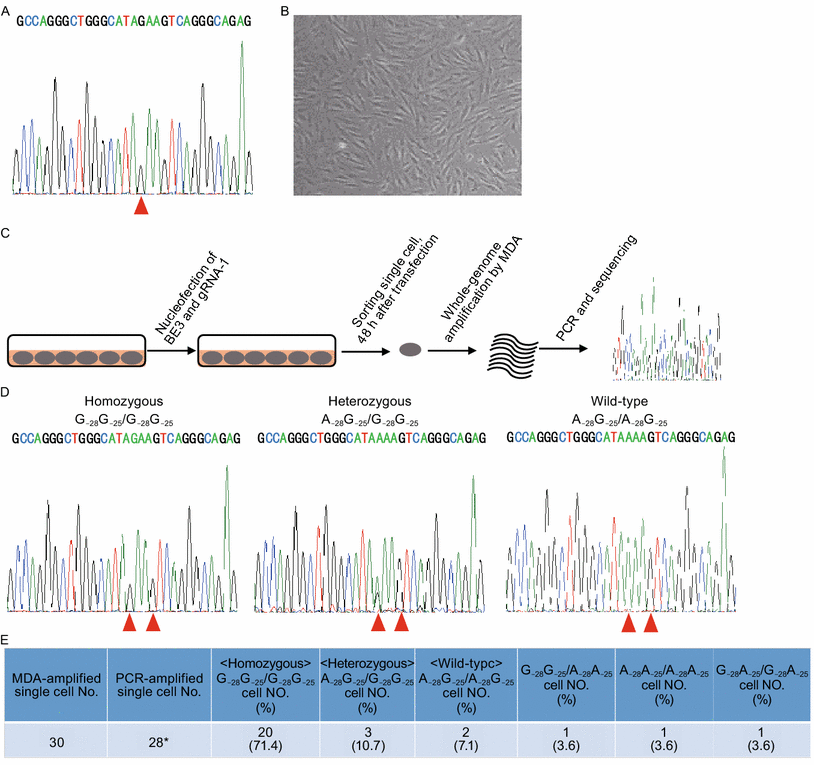
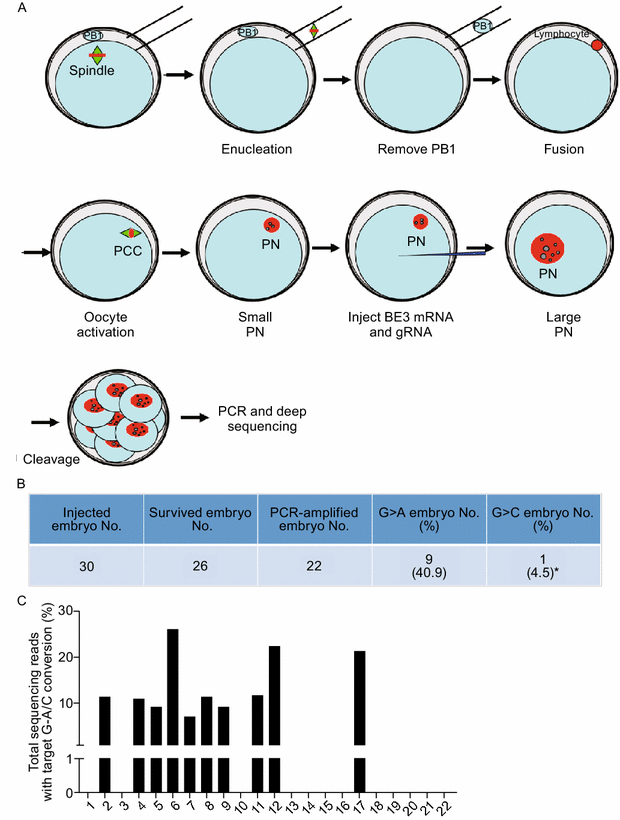
Image 3 below shows the final and third experiment applied on human embryo lab-developed with the infected gene by nuclear transfer, for the similar correction A>G at the HBB-28
Analysis of the data showed that G−28 was converted to either A or C in 45.4% (10/22) of the injected embryos. In embryo #17, G−28 was converted to C. In the other 9 embryos, G−28 was converted to A, representing precise mutation repairing
Is this a cure?
While at the moment the study could not report 100% accuracy, yet this is a good start for treating genetic disease, in light of prior research conducted earlier. Authors note:
Although we did not achieve 100% repair in human embryos, we and other groups have reported 100% base editing in mouse embryos (Kim et al., 2017c; Liang et al., 2017). By injecting BE3 protein and optimizing the injection time of the base editors, 100% repair of disease mutations may be achieved, as reported in CRISPR/Cas9 system(Hashimoto et al., 2016)
Ethics
While the topic of ethics in gene manipulation as well as using embryos for disease tests are always in effect, yet the study took specific care to avoid such collisions, received written approval from patients, donors, and the ethical community at Sun Yat-sen University, and while the process might still be frowned upon as a social-stigma, yet it definitely is crucial for future scientific improvements and growth.
Final findings
While this is great news and widens the doors for further work and research, still the statement by Darren Griffin, Professor of Genetics from the University of Kent is key to highlight the complexity of the process, and that this is merely the beginning of our progress:
it is important not to get carried away about its widespread utility if put into clinical practice. An embryo would still need to be diagnosed as abnormal (if there were other embryos in the cohort that were normal they would presumably be used instead), then the base editor applied, then re-diagnosed to make sure that it had worked. This would be an involved procedure that would be very expensive
For further in-depth details about the study and involved work, you can check out the full paper as reference 2.
Thank you for reading through!
References:
- http://www.iflscience.com/health-and-medicine/chemical-surgery-removes-inherited-disease-from-the-dna-of-human-embryos/all/
- https://link.springer.com/article/10.1007/s13238-017-0475-6 (Liang, P., Ding, C., Sun, H. et al. Protein Cell (2017). https://doi.org/10.1007/s13238-017-0475-6)
- http://www.independent.co.uk/news/science/chemical-surgery-human-embryos-disease-beta-thalassemia-base-editing-genetic-code-a7971441.html
- https://benchling.com/pub/liu-base-editor
Photo Credits:
- Image 1
- Remaing photos from original paper under Creative Commons Attribution 4.0 - Further credits:
No Changes were made to the original photos, which are copied and non-modified from original paper under reference 2 above.
Photos as well as research paper are all available under the terms of Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
A Proud member of MAP - Minnows Accelerator Project
MAP is a growing community helping talented minnows accelerate their growth on Steemit.
To join, check out the link at the home page of @accelerator account
One of my articles is featured in the MAP28 Contest "Six of the Best".
If you'd like to support me, please go to this post Minnow Accelerator Project MAP27 "Six of the Best" Contest. @accelerator/six-of-the-best-from-our-new-mapsters-map28-minnow-contest
And upvote my comment towards the bottom of the page, or just type the word "VOTE" under my comment.
Prior Posts:
If you enjoyed this post, you might want to check out some of my earlier posts:
- Nobel Prize: Discovery of "How" Humans Adapt Their Biological Rhythms to the Rotation of Planet Earth!
- Misconception #9: Color of Mucus Determines if you Need Antibiotics (And Some Drs Still Believe It)
- Daily Mysteries #4: What Causes Left Handedness ? (New Study Findings)
- Daily Mysteries #3: Are you Missing a Muscle?
- Are Viruses .. Alive?
- Daily Mysteries #2: Why do we dream?
- Astro News: Gravitational Waves Detected for the 4th Time by 3 Observatories
- Daily Mysteries #1: Why do we often forget what we just left the room to do?
- Misconception #8: Caesarian C-Section Is Named After Julius Caesar
- Misconception #7: Nero Set Rome On Fire And Watched It Burn
- Misconception #6: Low Voltage Shocks Are Not Dangerous
My posts aim to be contribute to the following projects:
- #steemSTEM / @steemstem - A project to increase both the quality as well as visibility of Science, Technology, Engineering and Mathematics (and Health).
- #steemiteducation / @steemiteducation - A project to promote high quality educational posts on Steemit.
