PART 3: ABSOLUTE ZERO IMPOSSIBLE

Absolute zero is the ultimate limit of cold, the temperature at which zero heat energy is present. It is the zero point of the ideal gas temperature scale, expressed as 0 degrees on the Kelvin (K) and Rankine (R) temperature scales. As a result of the third law of thermodynamics, the entropy of any object must apporach zero as its temperature approaches 0 K. We will discover some unbelievable properties of matter at low temperatures and why reaching absolute zero will forever remain impossible.
Temperature scales,
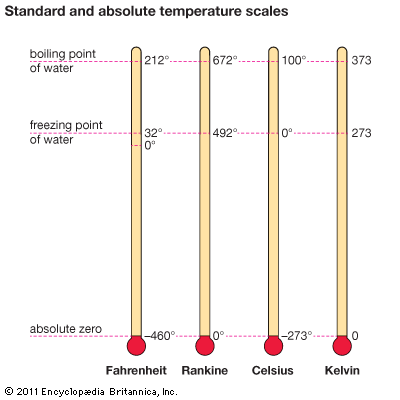
were introduced in the 1700-1800's. Ordinary temperature scales such as Fahrenheit or Celcius have an arbitrary zero point. The most common being the freezing or boiling point of water. On the other hand, an absolute temperature scale starts at absolute zero. There is an absolute limit of cold at around −273°C or 0 K. At this temperature, a system will be minimized by a unique minimum energy configuration. There is zero entropy, zero free energy, and all random molecular motion stops.
Compare these temperatures,
Kelvin versus the Farinhieght and Celcius scales you are familiar with.
| State | Kelvin | Celcius | Fahrenheit |
|---|---|---|---|
| Absolute zero | 0 K | -273 C | -460 F |
| Nitrogen boils | 77 K | -196 C | -321 F |
| Ice melts | 273 K | 0 C | 32 F |
| Room temperature | 300 K | 27 C | 80 F |
| Water boils | 373 K | 102 C | 212 F |
| Lead melts | 600 K | 327 C | 620 F |
| Surface of the Sun | 6000 K | 5727 C | 10340 F |
Over the last two centuries,
experimental physicists have produced colder and colder temperatures in their laboratories. With each breakthrough new gases, including oxygen, nitrogen, and hydrogen, were condensed into liquids.
In 1908, Heike Kamerlingh Onnes liquefied helium at a temperature of 5 K. Within a few years, physicists were able to produce temperatures below 1 degree above absolute zero.
Cooling a gas
is a basic process of alternating isothermal compressions and adiabatic expansions.
In isothermal compression, the gas is held at a constant temperature and slowly compressed.
In adiabatic expansion, the gas is expanded while insulated from its surroundings.
Since no heat is transferred, the entropy of the gas does not change.
The third law of thermodynamics,

discovered by Walther Nernst in 1905, prevents any object from being cooled to absolute zero. As any systems temperature approaches zero, entropy also approaches zero.
The cooling process can never reach absolute zero because heat can only be exchanged between two systems. The first a heat source is at a higher temperature and the other, the heat sink, is at a lower temperature. No heat sink below absolute zero could exist; thus it is impossible to reach absolute zero.
An analogy: It is impossible to dry off completely using towels that are slightly damp—you cannot get drier than the towel was when you started. Since everything starts out with at least a little heat energy, no finite process can wring every last bit of heat energy out of anything.
Absolute zero is impossible,
however physicists have come extremely close. Along the way, they have discovered some astounding new properties of matter.
In 1911, Kamerlingh Onnes discovered that at extremely low temperatures, metals can become superconductors, objects with zero electrical resistance, or electrical friction.
In 1937, Pyotr Kapitsa showed that liquid helium becomes a superfluid below 2 K. A superfluid has no viscosity, or flow friction, and perfect heat conduction.

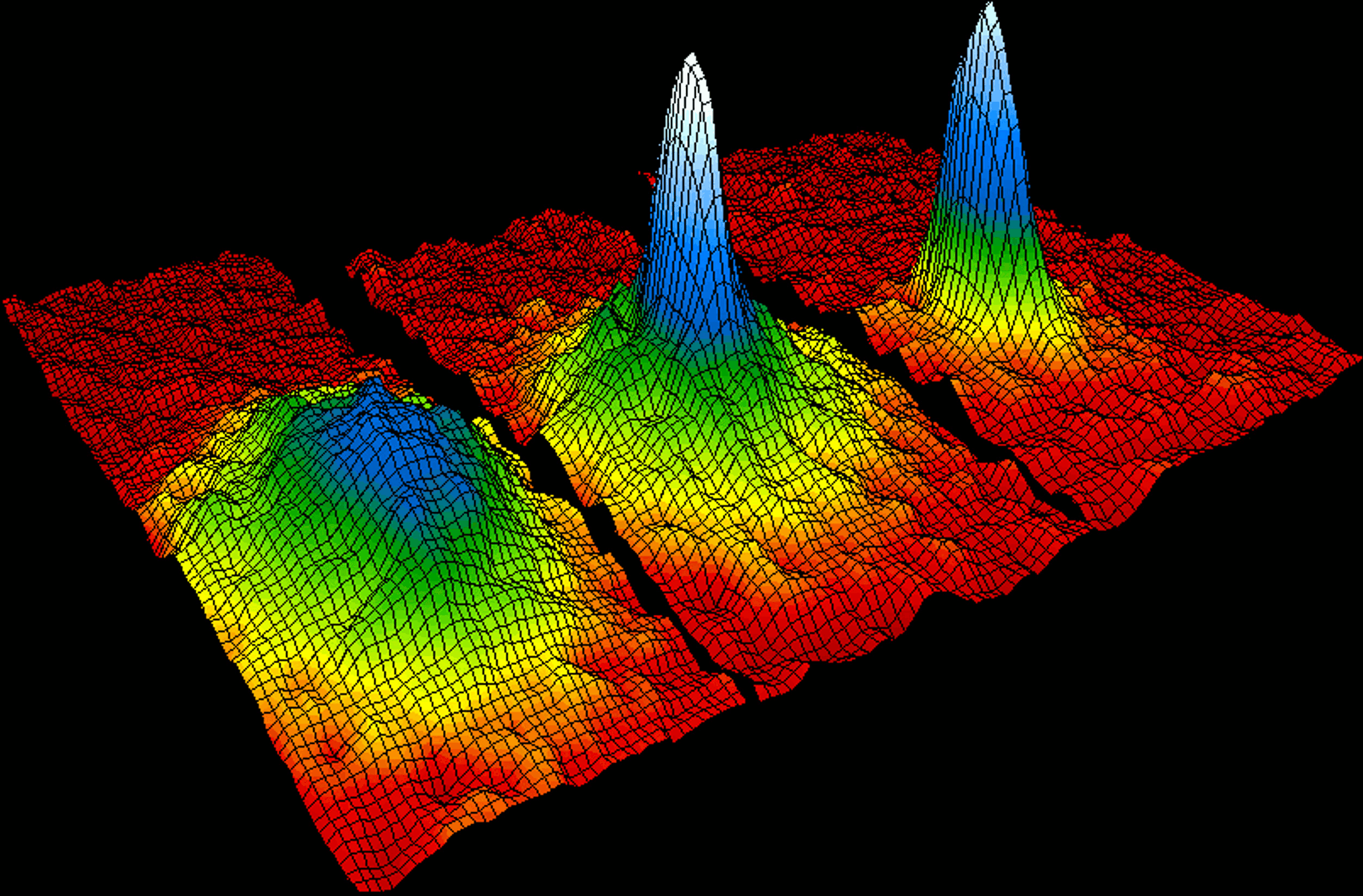
At less than one-millionth of a degree above absolute zero, a cloud of atoms can form a Bose-Einstein condensate; atoms lose their individuality and behave in a collective, quantum-mechanical way.
CONCLUSION
These new states of matter discovered at low temperatures are the result of quantum effects that can only be seen when there is very little heat energy present. As physicists learn to explore lower and lower temperatures new and exciting forms of matter may be discovered. It is important to remember no matter how advanced science gets our quest to reach absolute zero is impossible.
Questions to the reader (Answer in comments):
Consider the limitations we have learned about absolute minimum temperatures.
What possible limitations might there be that would define maximum possible temperature?
Impossible Science
#1 - THREE TYPES OF IMPOSSIBLE
#2 - ALMOST IMPOSSIBLE
#3 - ABSOLUTE ZERO IMPOSSIBLE