The dawn of the 20th century brought the biggest paradigm shift, to our web of belief in centuries.

German physicist Max Planck wondered about the relationship between heat and light. If light is a wave, he reasoned, then it should be able to have any strength—any amount of energy—yet it cannot.
Planck was forced to suppose that the energy of light comes in indivisible units or quanta.
Light is a wave, but it acts like it is made up of particles, which he called photons. He determined that the energy of the photon (E) is related to the frequency of the light wave (f): E = hf, where h is an extremely tiny number known as Planck’s constant. Ordinary light contains trillions and trillions of photons. Billions of photons are entering your eyes each second.
Albert Einstein read Planck’s idea and realized it could explain a major mystery of physics; the photoelectric effect.
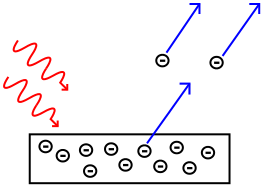
If we shine a light on a metal surface, electrons are ejected. Brighter light ejects more, but not more energetic, electrons. Einstein realized that the energy of the electrons is determined not by the light’s brightness but by its color, its frequency. Higher frequencies produce higher-energy electrons. This seems impossible if light is a wave. But it makes sense if we think of light as a stream of photons. Each photon gives its energy to one electron.
After Einstein’s work, quantum ideas began to spread throughout physics.

Physicists realized these strange ideas apply to matter as well. Danish physicist Niels Bohr suggested that quantum ideas determine the inner structure of atoms. According to 19th-century physics, orbiting electrons should radiate energy away, spiral inward, and collapse.
Bohr said that electrons can only orbit a nucleus at certain discrete distances determined by h.
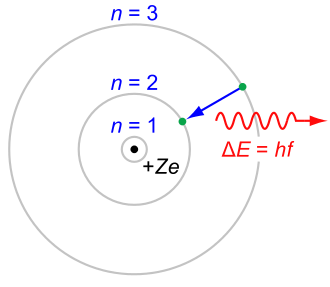
The lowest-energy electron has a minimum distance from the nucleus, so the atom cannot collapse. This idea, with some refinement, explains a lot of atomic properties, including the size of atoms.
Physicist than began to wonder if light was both a particle and a wave what about matter?
END PART 2
PART 1, PART 2, PART 3, PART 4
