In Newtonian Mechanics a particle can only pass through a barrier if it has enough kinetic energy to get over the bump in potential energy.
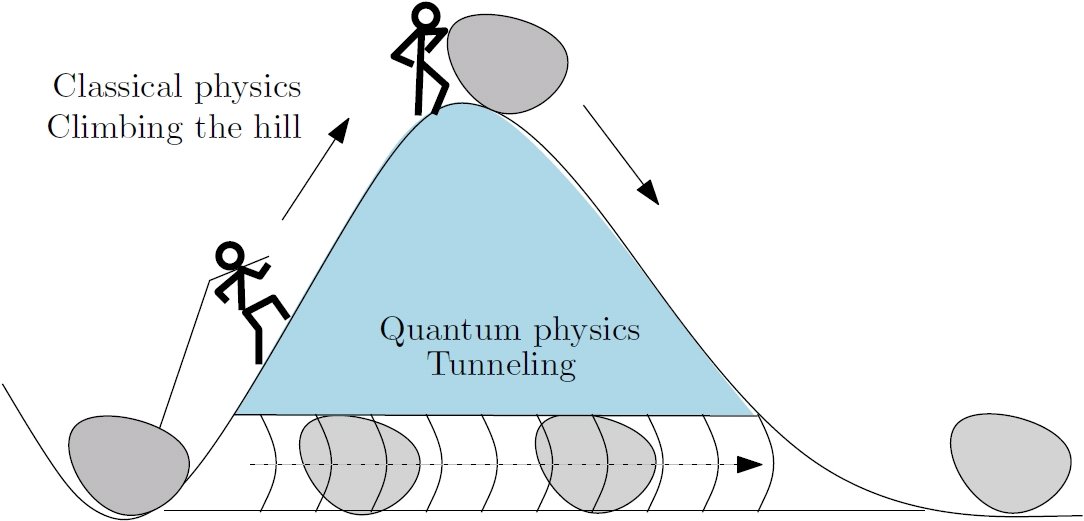
A quantum particle can sometimes sneak through a barrier even when its energy would seem to trap it on one side. This effect, called quantum tunneling, explains how trapped nuclear particles can sometimes escape their nuclei, leading to radioactive decay. Tunneling also allows photons of visible light to escape the interior of the sun and electrical currents to function.
To understand quantum tunneling, think about a particle moving on a line.
Now imagine placing a wall on each side of the particle
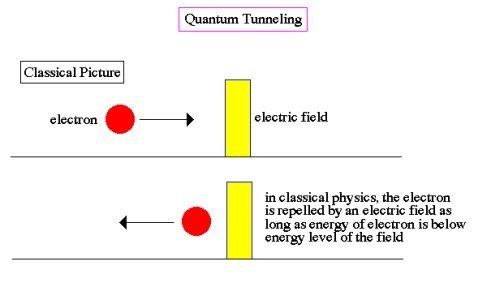
- In classical physics, the particle bounces back and forth between the walls and eventually stops, trapped. The particle has enough energy to be outside the walls, but it does not have enough energy to get there.
- In quantum mechanics, the particle behaves like a wave. The wave is most intense between the walls, so the particle is probably there. At the walls, the quantum wave diminishes but does not become zero; it extends slightly into the walls. A very low-intensity wave extends outside the walls. There is thus a tiny probability that the particle will be found outside the walls.
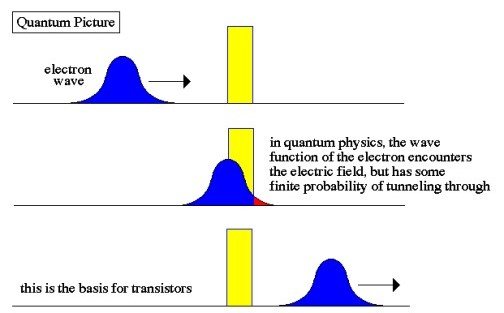
This chance to be found beyond the barrier is called the tunneling probability.
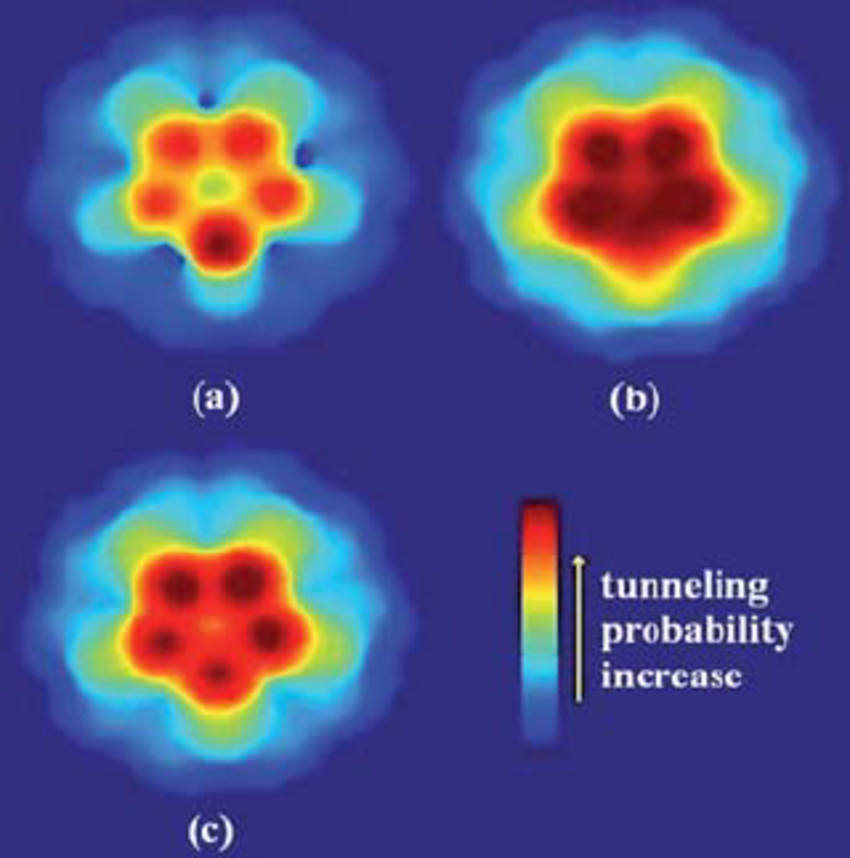
For a small particle and an atomic-sized barrier, the tunneling probability can be large; for a large object and a strong, thick barrier, the tunneling probability is extremely small and can border on statistically impossible.
Radioactive decay is a common example of quantum tunneling.
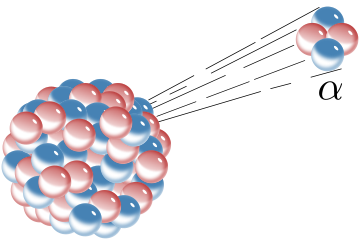
In alpha (α) decay, an unstable nucleus emits an alpha particle (containing 2 protons and 2 neutrons) that flies away, carrying energy.
The alpha (α) particle’s escape was first succesfully explained via tunneling by Russian physicist George Gamow.

- The nuclear forces are the potential energy barrier, binding the alpha (α) particle to the nucleus.
- But particle and nucleus are both positively charged, so electromagnetic repulsion pushes them apart.
- The probability of a particle escaping is the probability of the charge repulsion overcoming the nuclear force.
Electrical currents are also examples of quantum tunneling.
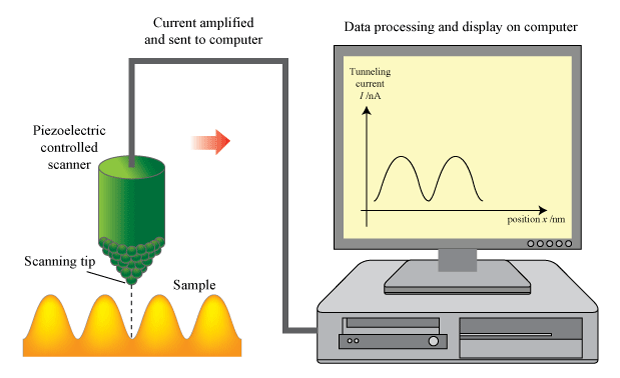
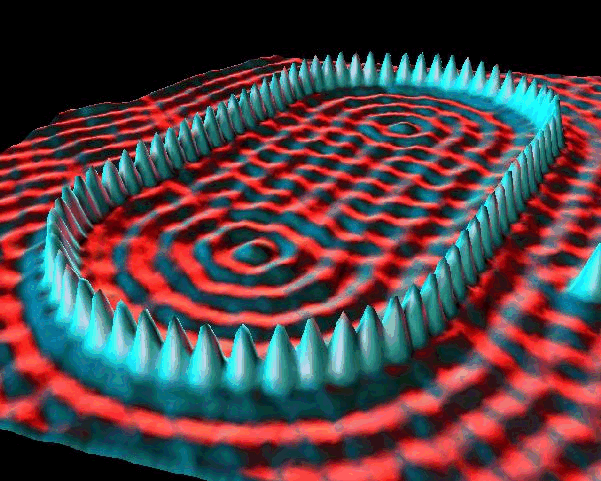
In a piece of solid graphite, electrons are trapped inside atoms. They have high potential energy and no kinetic energy. If you bring the tip of a thin wire very close to the surface, electrons can tunnel from the graphite to the wire, or vice versa. The smaller the gap, the more likely the tunneling. If you move the wire across the graphite and keep track of the number of electrons that tunnel through the gap, you can map out the bumps (smaller gaps) and hollows (larger gaps) in the graphite’s surface. This is the basis of the scanning tunneling microscope, a device for creating extremely detailed images of a surface.
Quantum tunneling is directly related to the uncertainty principle.
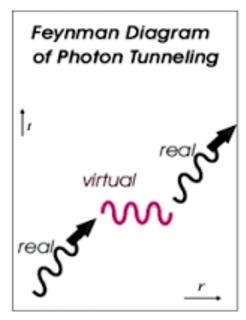
It is something like the creation and annihilation of a virtual particle in the quantum vacuum. The particle borrows the energy to get beyond the barrier, in effect, from nowhere. After the particle scoots past the barrier, it gives the energy back. If the time and the energy it takes to get past the barrier are small enough, the whole process is hidden by time-energy uncertainty: ∆t∆e > h.
Next time in Part 7: The most impassable barrier we have seen so far is the light cone. Can a particle use quantum tunneling to travel faster than light...?
End Part 6
PART 1, PART 2, PART 3, PART 4, PART 5, PART 6, PART 7
Image Credits:
http://www4.uwsp.edu/physastr/kmenning/images/corral2.gifhttps://kimminseodimension.files.wordpress.com/2014/04/image_127.jpghttp://www.physicsoftheuniverse.com/images/quantum_tunnelling.jpghttp://www.physicsoftheuniverse.com/images/quantum_tunnelling.jpghttps://www.researchgate.net/profile/Weitao_Zheng/publication/45279409/figure/fig8/AS:306090148417539@1449988986246/Fig-8-Tunneling-probability-patterns-for-HOMO-of-a-first-layer-B-doped-b.pnghttps://upload.wikimedia.org/wikipedia/commons/thumb/7/79/Alpha_Decay.svg/360px-Alpha_Decay.svg.pnghttp://www.azquotes.com/picture-quotes/quote-it-took-less-than-an-hour-to-make-the-atoms-a-few-hundred-million-years-to-make-the-george-gamow-75-51-69.jpghttp://www.hk-phy.org/atomic_world/stm/images/fig06.gif
